Effects of Resveratrol on the Renin-Angiotensin System in the Aging Kidney
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Animals
2.2. Evaluation of the Renal Function
2.3. Histological Assessment of the Renal Tissue
2.4. Immunohistochemistry
2.5. Western Blot Analysis
2.6. Enzyme Immunoassay
2.7. Statistical Analysis
3. Results
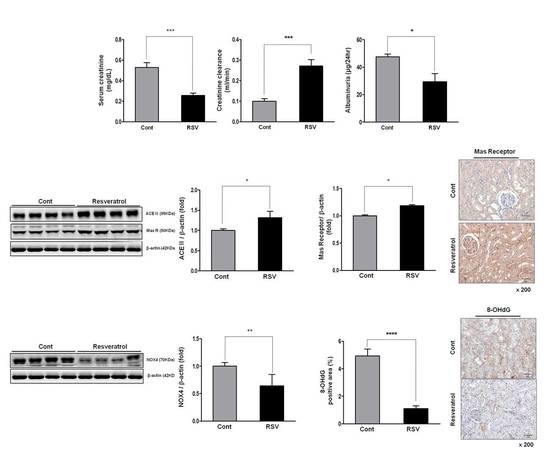
3.1. Effects of Resveratrol on Renal Function in Aging Mice
3.2. Effects of Resveratrol on the Renal Histological Changes in Aging Mice
3.3. Resveratrol Inhibits the Ang II/AT1R Axis in Aging Mice
3.4. Resveratrol Stimulates Angiotensin II Type 2 Receptors (AT2R) and Mas Receptor in Aging Mice
3.5. Effects of the Resveratrol on the Oxidative Stress Marker
3.6. Effects of Resveratrol on the Antioxidant Enzyme
3.7. Anti-Inflammatory Effects of Resveratrol
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Tosato, M.; Zamboni, V.; Ferrini, A.; Cesari, M. The aging process and potential interventions to extend life expectancy. Clin. Interv. Aging 2007, 2, 401–412. [Google Scholar] [PubMed]
- Denic, A.; Glassock, R.J.; Rule, A.D. Structural and functional changes with the aging kidney. Adv. Chronic Kidney Dis. 2016, 23, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Ozieh, M.N.; Bishu, K.G.; Dismuke, C.E.; Egede, L.E. Trends in healthcare expenditure in united states adults with chronic kidney disease: 2002–2011. BMC Health Serv. Res. 2017, 17, 368. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Mason, A. Cost of aging. Financ. Dev. 2017, 54, 7–9. [Google Scholar]
- Kovacic, J.C.; Moreno, P.; Nabel, E.G.; Hachinski, V.; Fuster, V. Cellular senescence, vascular disease, and aging: Part 2 of a 2-part review: Clinical vascular disease in the elderly. Circulation 2011, 123, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, E.D.; Hughes, J.; Ferenbach, D.A. Renal aging: Causes and consequences. J. Am. Soc. Nephrol. 2017, 28, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Capettini, L.S.; Montecucco, F.; Mach, F.; Stergiopulos, N.; Santos, R.A.; da Silva, R.F. Role of renin-angiotensin system in inflammation, immunity and aging. Curr. Pharm. Des. 2012, 18, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Conti, S.; Cassis, P.; Benigni, A. Aging and the renin-angiotensin system. Hypertension 2012, 60, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.A.; Ferreira, A.J.; Verano-Braga, T.; Bader, M. Angiotensin-converting enzyme 2, angiotensin-(1-7) and mas: New players of the renin-angiotensin system. J. Endocrinol. 2013, 216, R1–R17. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lo, C.S.; Padda, R.; Abdo, S.; Chenier, I.; Filep, J.G.; Ingelfinger, J.R.; Zhang, S.L.; Chan, J.S. Angiotensin-(1-7) prevents systemic hypertension, attenuates oxidative stress and tubulointerstitial fibrosis, and normalizes renal angiotensin-converting enzyme 2 and mas receptor expression in diabetic mice. Clin. Sci. 2015, 128, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Chappell, M.C. Emerging evidence for a functional angiotensin-converting enzyme 2-angiotensin-(1-7)-mas receptor axis: More than regulation of blood pressure? Hypertension 2007, 50, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahab, A.; Gao, K.; Jia, C.; Zhang, F.; Tian, G.; Murtaza, G.; Chen, J. Significance of resveratrol in clinical management of chronic diseases. Molecules 2017, 22, 1329. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Koya, D. Renal protective effects of resveratrol. Oxid. Med. Cell. Longev. 2013, 2013, 568093. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Huang, X.; Zhang, M.; Zhang, L.; Chen, J.; Gu, Y.; Hao, C.M. Resveratrol attenuates diabetic nephropathy via modulating angiogenesis. PLoS ONE 2013, 8, e82336. [Google Scholar] [CrossRef] [PubMed]
- Albertoni, G.; Schor, N. Resveratrol plays important role in protective mechanisms in renal disease-mini-review. Braz. J. Nephrol. 2015, 37, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Namkoong, K.; Shin, M.; Park, J.; Yang, E.; Ihm, J.; Thu, V.T.; Kim, H.K.; Han, J. Cardiovascular protective effects and clinical applications of resveratrol. J. Med. Food 2017, 20, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Jang, I.-A.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging 2018, 10, 83–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baur, J.A.; Ungvari, Z.; Minor, R.K.; Le Couteur, D.G.; De Cabo, R. Are sirtuins viable targets for improving healthspan and lifespan? Nat. Rev. Drug Discov. 2012, 11, 443–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozbek, E. Induction of oxidative stress in kidney. Int. J. Nephrol. 2012, 2012, 465897. [Google Scholar] [CrossRef] [PubMed]
- Rhee, H.; Han, M.; Kim, S.S.; Kim, I.Y.; Lee, H.W.; Bae, S.S.; Ha, H.K.; Jung, E.S.; Lee, M.Y.; Seong, E.Y. The expression of two isoforms of matrix metalloproteinase-2 in aged mouse models of diabetes mellitus and chronic kidney disease. Kidney Res. Clin. Pract. 2018, 37, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Jin, K. Modern biological theories of aging. Aging Dis. 2010, 1, 72–74. [Google Scholar] [PubMed]
- Sergiev, P.; Dontsova, O.; Berezkin, G. Theories of aging: An ever-evolving field. Acta Nat. 2015, 7, 9–18. [Google Scholar]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid. Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.E.; Choi, B.S. The renin-angiotensin system and aging in the kidney. Korean J. Intern. Med. 2014, 29, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Musso, C.G.; Jauregui, J.R. Renin-angiotensin-aldosterone system and the aging kidney. Expert Rev. Endocrinol. Metab. 2014, 9, 543–546. [Google Scholar] [CrossRef]
- Benigni, A.; Cassis, P.; Remuzzi, G. Angiotensin ii revisited: New roles in inflammation, immunology and aging. EMBO Mol. Med. 2010, 2, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Diz, D.I.; Lewis, K. Dahl memorial lecture: The renin-angiotensin system and aging. Hypertension 2008, 52, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Gilliam-Davis, S.; Payne, V.S.; Kasper, S.O.; Tommasi, E.N.; Robbins, M.E.; Diz, D.I. Long-term at1 receptor blockade improves metabolic function and provides renoprotection in fischer-344 rats. Am. J. Physiol.-Heart Circ. Physiol. 2007, 293, H1327–H1333. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Xue, L.-J.; Yang, Y.; Wang, Q.-G.; Xue, X.; Ou, Z.; Gao, Q.; Shi, J.-Q.; Wu, L.; Zhang, Y.-D. Ave0991, a nonpeptide analogue of ang-(1-7), attenuates aging-related neuroinflammation. Aging 2018, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Pena Silva, R.A.; Chu, Y.; Miller, J.D.; Mitchell, I.J.; Penninger, J.M.; Faraci, F.M.; Heistad, D.D. Impact of ace2 deficiency and oxidative stress on cerebrovascular function with aging. Stroke 2012, 43, 3358–3363. [Google Scholar] [CrossRef] [PubMed]
- Bruce, E.B.; Sakarya, Y.; Kirichenko, N.; Toklu, H.Z.; Sumners, C.; Morgan, D.; Tumer, N.; Scarpace, P.J.; Carter, C.S. Ace2 activator diminazene aceturate reduces adiposity but preserves lean mass in young and old rats. Exp. Gerontol. 2018, 111, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Weidmann, P.; De Myttenaere-Bursztein, S.; Maxwell, M.H.; de Lima, J. Effect of aging on plasma renin and aldosterone in normal man. Kidney Int. 1975, 8, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Jung, F.F.; Kennefick, T.M.; Ingelfinger, J.R.; Vora, J.P.; Anderson, S. Down-regulation of the intrarenal renin-angiotensin system in the aging rat. J. Am. Soc. Nephrol. 1995, 5, 1573–1580. [Google Scholar] [PubMed]
- Tank, J.E.; Vora, J.P.; Houghton, D.C.; Anderson, S. Altered renal vascular responses in the aging rat kidney. Am. J. Physiol.-Ren. Physiol. 1994, 266, F942–F948. [Google Scholar] [CrossRef] [PubMed]
- Musso, C.G.; Oreopoulos, D.G. Aging and physiological changes of the kidneys including changes in glomerular filtration rate. Nephron Physiol. 2011, 119 (Suppl. 1), p1–p5. [Google Scholar] [CrossRef]
- Dinh, Q.N.; Drummond, G.R.; Kemp-Harper, B.K.; Diep, H.; Silva, T.M.D.; Kim, H.A.; Vinh, A.; Robertson, A.A.B.; Cooper, M.A.; Mansell, A.; et al. Pressor response to angiotensin ii is enhanced in aged mice and associated with inflammation, vasoconstriction and oxidative stress. Aging 2017, 9, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.E.; Kim, E.N.; Kim, M.Y.; Lim, J.H.; Jang, I.A.; Ban, T.H.; Shin, S.J.; Park, C.W.; Chang, Y.S.; Choi, B.S. Age-associated changes in the vascular renin-angiotensin system in mice. Oxid. Med. Cell. Longev. 2016, 2016, 6731093. [Google Scholar] [CrossRef] [PubMed]
- Labinskyy, N.; Csiszar, A.; Veress, G.; Stef, G.; Pacher, P.; Oroszi, G.; Wu, J.; Ungvari, Z. Vascular dysfunction in aging: Potential effects of resveratrol, an anti-inflammatory phytoestrogen. Curr. Med. Chem. 2006, 13, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, T.; Miyazaki, R.; Kamiharaguchi, A.; Hashimoto, T.; Matsuura, H.; Kitamoto, S.; Tokunou, T.; Sunagawa, K. Resveratrol attenuates angiotensin ii-induced senescence of vascular smooth muscle cells. Regul. Pept. 2012, 177, 35–39. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Vitiello, M.; Casale, R.; Servillo, L.; Giovane, A.; Balestrieri, M.L. Sirtuins in vascular diseases: Emerging roles and therapeutic potential. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2015, 1852, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Kume, S.; Takeda-Watanabe, A.; Kanasaki, K.; Koya, D. Sirtuins and renal diseases: Relationship with aging and diabetic nephropathy. Clin. Sci. 2013, 124, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Hamza, S.M.; Dyck, J.R. Systemic and renal oxidative stress in the pathogenesis of hypertension: Modulation of long-term control of arterial blood pressure by resveratrol. Front. Physiol. 2014, 5, 292. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, C.W. Adenosine monophosphate–activated protein kinase in diabetic nephropathy. Kidney Res. Clin. Pract. 2016, 35, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, R.; Ichiki, T.; Hashimoto, T.; Inanaga, K.; Imayama, I.; Sadoshima, J.; Sunagawa, K. Sirt1, a longevity gene, downregulates angiotensin ii type 1 receptor expression in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.N.; Kim, M.Y.; Lim, J.H.; Kim, Y.; Shin, S.J.; Park, C.W.; Kim, Y.S.; Chang, Y.S.; Yoon, H.E.; Choi, B.S. The protective effect of resveratrol on vascular aging by modulation of the renin-angiotensin system. Atherosclerosis 2018, 270, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Sachse, A.; Wolf, G. Angiotensin ii–induced reactive oxygen species and the kidney. J. Am. Soc. Nephrol. 2007, 18, 2439–2446. [Google Scholar] [CrossRef] [PubMed]
- Montezano, A.C.; Nguyen Dinh Cat, A.; Rios, F.J.; Touyz, R.M. Angiotensin ii and vascular injury. Curr. Hypertens. Rep. 2014, 16, 431. [Google Scholar] [CrossRef] [PubMed]
- Sedeek, M.; Nasrallah, R.; Touyz, R.M.; Hebert, R.L. Nadph oxidases, reactive oxygen species, and the kidney: Friend and foe. J. Am. Soc. Nephrol. 2013, 24, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.S.; Wilcox, C.S. Nadph oxidases in the kidney. Antioxid. Redox Signal. 2006, 8, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Oudot, A.; Vergely, C.; Ecarnot-Laubriet, A.; Rochette, L. Angiotensin ii activates nadph oxidase in isolated rat hearts subjected to ischaemia–reperfusion. Eur. J. Pharmacol. 2003, 462, 145–154. [Google Scholar] [CrossRef]
- Garrido, A.M.; Griendling, K.K. Nadph oxidases and angiotensin ii receptor signaling. Mol. Cell. Endocrinol. 2009, 302, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, G.; Stopper, H.; Schinzel, R.; Ni, C.W.; Jo, H.; Schupp, N. Angiotensin ii induces DNA damage via at1 receptor and nadph oxidase isoform nox4. Mutagenesis 2012, 27, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Oudot, A.; Martin, C.; Busseuil, D.; Vergely, C.; Demaison, L.; Rochette, L. Nadph oxidases are in part responsible for increased cardiovascular superoxide production during aging. Free Radic. Biol. Med. 2006, 40, 2214–2222. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Dinh Cat, A.; Montezano, A.C.; Burger, D.; Touyz, R.M. Angiotensin ii, nadph oxidase, and redox signaling in the vasculature. Antioxid. Redox Signal. 2013, 19, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Munzel, T.; Camici, G.G.; Maack, C.; Bonetti, N.R.; Fuster, V.; Kovacic, J.C. Impact of oxidative stress on the heart and vasculature: Part 2 of a 3-part series. J. Am. Coll. Cardiol. 2017, 70, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.; Patel, V.B.; Wang, Z.; Levasseur, J.; Kaufman, S.; Penninger, J.M.; Oudit, G.Y. Angiotensin-converting enzyme 2 antagonizes angiotensin ii-induced pressor response and nadph oxidase activation in wistar-kyoto rats and spontaneously hypertensive rats. Exp. Physiol. 2013, 98, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Tanno, T.; Tomita, H.; Narita, I.; Kinjo, T.; Nishizaki, K.; Ichikawa, H.; Kimura, Y.; Tanaka, M.; Osanai, T.; Okumura, K. Olmesartan inhibits cardiac hypertrophy in mice overexpressing renin independently of blood pressure: Its beneficial effects on ace2/ang (1–7)/mas axis and nadph oxidase expression. J. Cardiovasc. Pharmacol. 2016, 67, 503–509. [Google Scholar] [CrossRef] [PubMed]













© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, I.-A.; Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Effects of Resveratrol on the Renin-Angiotensin System in the Aging Kidney. Nutrients 2018, 10, 1741. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10111741
Jang I-A, Kim EN, Lim JH, Kim MY, Ban TH, Yoon HE, Park CW, Chang YS, Choi BS. Effects of Resveratrol on the Renin-Angiotensin System in the Aging Kidney. Nutrients. 2018; 10(11):1741. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10111741
Chicago/Turabian StyleJang, In-Ae, Eun Nim Kim, Ji Hee Lim, Min Young Kim, Tae Hyun Ban, Hye Eun Yoon, Cheol Whee Park, Yoon Sik Chang, and Bum Soon Choi. 2018. "Effects of Resveratrol on the Renin-Angiotensin System in the Aging Kidney" Nutrients 10, no. 11: 1741. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10111741