In Vitro Hypocholesterolemic Effect of Coffee Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample and Extracts Preparation
2.2. Sugar Analysis
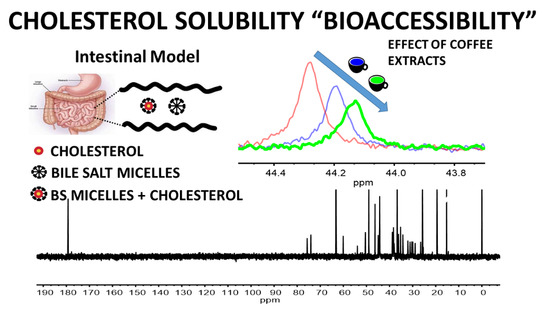
2.3. In Vitro Cholesterol Solubility Assay
2.4. Determination of the Particles Size in Solution
2.5. Determination of Crystals Thermotropic Behavior
2.6. Statistics Analysis
3. Results and Discussion
3.1. Sugars Analysis
3.2. Cholesterol Bioaccessibility and Bile Salt Sequestration
3.2.1. Effect of Coffee Extracts
3.2.2. Effect of Polysaccharides AG and GM Extracted from Coffee
3.2.3. Effect of Lipid Fraction Extracted from Coffee
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holst, M.; Maxwell, D.; Mazzeo, R. Conformal Fields and the Structure of the Space of Solutions of the Einstein Constraint Equations. Annu. Rev. Biophys. Biomol. Struct. 2017, 33, 269–295. [Google Scholar]
- Patton, J.S.; Carey, M.C. Watching fat digestion. Science 1979, 204, 145–148. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, N.G. Digestion and absorption. Anaesth. Intensive Care Med. 2018, 19, 87–92. [Google Scholar] [CrossRef]
- Donovan, J.M.; Timofeyeva, N.; Carey, M.C. Influence of total lipid concentration, bile salt:lecithin ratio, and cholesterol content on inter-mixed micellar/vesicular (non-lecithin-associated) bile salt concentrations in model bile. J. Lipid Res. 1991, 32, 1501–1512. [Google Scholar]
- Ferreira, T.M.; Coreta-Gomes, F.; Samuli Ollila, O.H.; Moreno, M.J.; Vaz, W.L.C.; Topgaard, D. Cholesterol and POPC segmental order parameters in lipid membranes: Solid state1H-13C NMR and MD simulation studies. Phys. Chem. Chem. Phys. 2013, 15, 1976–1989. [Google Scholar] [CrossRef]
- Ardies, C.M.; Roberts, C.K. Atherosclerosis. Diet. Exerc. Chronic Dis. Biol. Basis Prev. 2014, 407, 133–210. [Google Scholar]
- West, R.S.C. Pathogenesis of Atherosclerosis. J. Am. Coll. Cardiol. 2006, 47, C7–C12. [Google Scholar]
- World Health Organization. World Health Statistics: Monitoring Health for The SDGs; World Health Organization: Geneva, Switzerland, 2017; ISBN 9788578110796. [Google Scholar]
- Hui, D.Y.; Howles, P.N. Molecular mechanisms of cholesterol absorption and transport in the intestine. Semin. Cell Dev. Biol. 2005, 16, 183–192. [Google Scholar] [CrossRef]
- Grundy, S.M.; Ahrens, E.H.; Davignon, J.; Ahrens, E.H., Jr. The interaction of cholesterol absorption and cholesterol synthesis in man. J. Lipid Res. 1969, 10, 304–315. [Google Scholar]
- Ostlund, R.E. Cholesterol absorption. Curr. Opin. Gastroenterol. 2002, 18, 254–258. [Google Scholar] [CrossRef]
- Out, C.; Groen, A.K.; Brufau, G. Bile acid sequestrants: More than simple resins. Curr. Opin. Lipidol. 2012, 23, 43–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahouny, G.V.; Tombes, R.; Cassidy, M.M.; Kritchevsky, D.; Gallo, L.L. Dietary fibers: V. Binding of bile salts, phospholipids and cholesterol from mixed micelles by bile acid sequestrants and dietary fibers. Lipids 1980, 15, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Heřmánková, E.; Žák, A.; Poláková, L.; Hobzová, R.; Hromádka, R.; Širc, J. Polymeric bile acid sequestrants: Review of design, in vitro binding activities, and hypocholesterolemic effects. Eur. J. Med. Chem. 2018, 144, 300–317. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA) Scientific Opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood LDL-cholesterol concentrations. Efsa 2011, 9, 2207.
- Chen, Z.Y.; Jiao, R.; Ka, Y.M. Cholesterol-lowering nutraceuticals and functional foods. J. Agric. Food Chem. 2008, 56, 8761–8773. [Google Scholar] [CrossRef] [PubMed]
- Viebke, C.; Al-Assaf, S.; Phillips, G.O. Food hydrocolloids and health claims. Bioact. Carbohydrates Diet. Fibre 2014, 4, 101–114. [Google Scholar] [CrossRef]
- Lopez-Huertas, E. Health effects of oleic acid and long chain omega-3 fatty acids (EPA and DHA) enriched milks. A review of intervention studies. Pharmacol. Res. 2010, 61, 200–207. [Google Scholar] [CrossRef]
- Coreta-Gomes, F.M.; Vaz, W.L.C.; Wasielewski, E.; Geraldes, C.F.G.C.; Moreno, M.J. Quantification of cholesterol solubilized in bile salt micellar aqueous solutions using 13C nuclear magnetic resonance. Anal. Biochem. 2012, 427, 41–48. [Google Scholar] [CrossRef]
- Coreta-Gomes, F.M.; Vaz, W.L.C.; Wasielewski, E.; Geraldes, C.F.G.; Moreno, M.J. Quantification of cholesterol solubilized in dietary micelles: Dependence on human bile salt variability and the presence of dietary food ingredients. Langmuir 2016, 32, 4564–4574. [Google Scholar] [CrossRef]
- Theuwissen, E.; Mensink, R.P. Water-soluble dietary fibers and cardiovascular disease. Physiol. Behav. 2008, 94, 285–292. [Google Scholar] [CrossRef]
- Rideout, T.C.; Harding, S.V.; Jones, P.J.; Fan, M.Z. Guar gum and similar soluble fibers in the regulation of cholesterol metabolism: Current understandings and future research priorities. Vasc. Health Risk Manag. 2008, 4, 1023–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendall, C.W.C.; Esfahani, A.; Jenkins, D.J.A. The link between dietary fibre and human health. Food Hydrocoll. 2010, 24, 42–48. [Google Scholar] [CrossRef]
- Cohn, J.S.; Kamili, A.; Wat, E.; Chung, R.W.S.; Tandy, S. Reduction in intestinal cholesterol absorption by various food components: Mechanisms and implications. Atheroscler. Suppl. 2010, 11, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.L.; Anderson, J.W.; Jennings, D. Propionate May Mediate the Hypocholesterolemic Effects of Certain Soluble Plant Fibers in Cholesterol-Fed Rats. Proc. Soc. Exp. Biol. Med. 1984, 175, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F.; Theuwissen, E.; Adam, A.; Bell, M.; Berger, A.; Mensink, R.P. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. Eur. J. Clin. Nutr. 2012, 66, 591–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasquier, B.; Armand, M.; Guillon, F.; Castelain, C.; Borel, P.; Barry, J.-L.; Pleroni, G.; Lairon, D. Viscous soluble dietary fibers alter emulsification and lipolysis of triacylglycerols in duodenal medium in vitro. J. Nutr. Biochem. 1996, 7, 293–302. [Google Scholar] [CrossRef]
- Topping, D.L. Soluble Fiber Polysaccharides: Effects on Plasma Cholesterol and Colonic Fermentation. Nutr. Rev. 1991, 49, 195–203. [Google Scholar] [CrossRef]
- McRorie, J.W.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabek, H.; Messerschmidt, S.; Brulport, V.; Goff, H.D. The effect of invitro digestive processes on the viscosity of dietary fibres and their influence on glucose diffusion. Food Hydrocoll. 2014, 35, 718–726. [Google Scholar] [CrossRef]
- Brownlee, I.A. The physiological roles of dietary fibre. Food Hydrocoll. 2011, 25, 238–250. [Google Scholar] [CrossRef]
- Lopes, G.R.; Ferreira, A.S.; Pinto, M.; Passos, C.P.; Coelho, E.; Rodrigues, C.; Figueira, C.; Rocha, S.M.; Nunes, F.M.; Coimbra, M.A. Carbohydrate content, dietary fibre and melanoidins: Composition of espresso from single-dose coffee capsules. Food Res. Int. 2016, 89, 989–996. [Google Scholar] [CrossRef]
- Moreira, A.S.P.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. Food Funct. 2012, 3, 903. [Google Scholar] [CrossRef] [PubMed]
- Simões, J.; Nunes, F.M.; Domingues, M.; do, R.M.; Coimbra, M.A. Structural features of partially acetylated coffee galactomannans presenting immunostimulatory activity. Carbohydr. Polym. 2010, 79, 397–402. [Google Scholar] [CrossRef]
- Passos, C.P.; Cepeda, M.R.; Ferreira, S.S.; Nunes, F.M.; Evtuguin, D.V.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Influence of molecular weight on in vitro immunostimulatory properties of instant coffee. Food Chem. 2014, 161, 60–66. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Cepeda, M.R.; Lopes, G.R.; Teixeira-Coelho, M.; Madureira, P.; Nunes, F.M.; Vilanova, M.; Coimbra, M.A. Structural polymeric features that contribute to in vitro immunostimulatory activity of instant coffee. Food Chem. 2018, 242, 548–554. [Google Scholar] [CrossRef]
- Moreira, A.S.P.; Coimbra, M.A.; Nunes, F.M.; Simões, J.; Domingues, M.R.M. Evaluation of the effect of roasting on the structure of coffee galactomannans using model oligosaccharides. J. Agric. Food Chem. 2011, 59, 10078–10087. [Google Scholar] [CrossRef]
- Morales, F.J.; Somoza, V.; Fogliano, V. Physiological relevance of dietary melanoidins. Amino Acids 2012, 42, 1097–1109. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Morales, F.J. Unraveling the contribution of melanoidins to the antioxidant activity of coffee brews. J. Agric. Food Chem. 2005, 53, 1403–1407. [Google Scholar] [CrossRef] [Green Version]
- Sridevi, V.; Giridhar, P.; Ravishankar, G.A. Evaluation of Roasting and Brewing effect on Antinutritional Diterpenes-Cafestol and Kahweol in Coffee. Glob. J. Med. Res. 2011, 11, 1–7. [Google Scholar]
- Moeenfard, M.; Erny, G.L.; Alves, A. Variability of some diterpene esters in coffee beverages as influenced by brewing procedures. J. Food Sci. Technol. 2016, 53, 3916–3927. [Google Scholar] [CrossRef] [Green Version]
- Haseeb, S.; Alexander, B.; Baranchuk, A. Wine and Cardiovascular Health. Circulation 2017, 136, 1434–1448. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.R.; Passos, C.P.; Rodrigues, C.; Teixeira, J.A.; Coimbra, M.A. Modulation of infusion processes to obtain coffee-derived food ingredients with distinct composition. Eur. Food Res. Technol. 2019, 245, 2133–2146. [Google Scholar] [CrossRef] [Green Version]
- Lopes, G.R.; Passos, C.P.; Rodrigues, C.; Teixeira, J.A.; Coimbra, M.A. Impact of microwave-assisted extraction on roasted coffee carbohydrates, caffeine, chlorogenic acids and coloured compounds. Food Res. Int. 2020, 129, 108864. [Google Scholar] [CrossRef]
- Passos, C.P.; Moreira, A.S.P.; Domingues, M.R.M.; Evtuguin, D.V.; Coimbra, M.A. Sequential microwave superheated water extraction of mannans from spent coffee grounds. Carbohydr. Polym. 2014, 103, 333–338. [Google Scholar] [CrossRef]
- Barbosa, H.M.A.; De Melo, M.M.R.; Coimbra, M.A.; Passos, C.P.; Silva, C.M. Optimization of the supercritical fluid coextraction of oil and diterpenes from spent coffee grounds using experimental design and response surface methodology. J. Supercrit. Fluids 2014, 85, 165–172. [Google Scholar] [CrossRef]
- Passos, C.P.; Coimbra, M.A. Microwave superheated water extraction of polysaccharides from spent coffee grounds. Carbohydr. Polym. 2013, 94, 626–633. [Google Scholar] [CrossRef]
- Santos, N.C.; Castanho, M.A. Teaching Light Scattering Spectroscopy: The Dimension and Shape of Tobacco Mosaic Virus. Biophys. J. 1996, 71, 1641–1650. [Google Scholar] [CrossRef] [Green Version]
- Passos, C.P.; Rudnitskaya, A.; Neves, J.M.M.G.C.; Lopes, G.R.; Evtuguin, D.V.; Coimbra, M.A. Structural features of spent coffee grounds water-soluble polysaccharides: Towards tailor-made microwave assisted extractions. Carbohydr. Polym. 2019, 214, 53–61. [Google Scholar] [CrossRef]
- Nunes, F.M.; Coimbra, M.A. Chemical Characterization of the High Molecular Weight Material Extracted with Hot Water from Green and Roasted Arabica Coffee. J. Agric. Food Chem. 2001, 49, 1773–1782. [Google Scholar] [CrossRef]
- Staggers, J.E.; Hernell, O.; Stafford, R.J.; Carey, M.C. Physical-chemical behavior of dietary and biliary lipids during intestinal digestion and absorption. 1. Phase behavior and aggregation states of model lipid systems patterned after aqueous duodenal contents of healthy adult human beings. Biochemistry 1990, 29, 2028–2040. [Google Scholar] [CrossRef]
- Hernell, O.; Staggers, J.E.; Carey, M.C. Physical-Chemical Behavior of Dietary and Biliary Lipids during Intestinal Digestion and Absorption. 2. Phase Analysis and Aggregation States of Luminal Lipids during Duodenal Fat Digestion in Healthy Adult Human Beings. Biochemistry 1990, 29, 2041–2056. [Google Scholar] [CrossRef] [PubMed]
- Carey, M.C.; Martin, C.; Carey, M.C. Micelle Formation by Bile Salts. Arch. Intern. Med. 2012, 130, 506. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- Salvioli, G.; Igimi, H.; Carey, M.C. Cholesterol gallstone dissolution in bile. Dissolution kinetics of crystalline cholesterol monohydrate by conjugated chenodeoxycholate-lecithin and conjugated ursodeoxycholate-lecithin mixtures: Dissimilar phase equilibria and dissolution mechanisms. J. Lipid Res. 1983, 24, 701–720. [Google Scholar] [PubMed]
- Gloess, A.N.; Schönbächler, B.; Klopprogge, B.; D’Ambrosio, L.; Chatelain, K.; Bongartz, A.; Strittmatter, A.; Rast, M.; Yeretzian, C. Comparison of nine common coffee extraction methods: Instrumental and sensory analysis. Eur. Food Res. Technol. 2013, 236, 607–627. [Google Scholar] [CrossRef] [Green Version]
- De Melo, M.M.R.; Barbosa, H.M.A.; Passos, C.P.; Silva, C.M. Supercritical fluid extraction of spent coffee grounds: Measurement of extraction curves, oil characterization and economic analysis. J. Supercrit. Fluids 2014, 86, 150–159. [Google Scholar] [CrossRef]
- Ratnayake, W.M.N.; Hollywood, R.; O’Grady, E.; Stavric, B. Lipid content and composition of coffee brews prepared by different methods. Food Chem. Toxicol. 1993, 31, 263–269. [Google Scholar] [CrossRef]





| Coffee Samples (S) and Extracts (E) | ||
|---|---|---|
| Designation | Operating conditions | |
| S1 (Decaff) | Coffee pod machine extraction (19 bar, ratio 6 g: 40 mL) | |
| S2 | ||
| S3 | ||
| E1 | Non-pressurized | RGC, Solid/liquid extraction (6 g: 30 mL, 20 °C, 10 min) |
| E2 | RGC, Solid/liquid extraction (6 g: 30 mL, 20 °C, 360 min) | |
| E3 | Pressurized | RGC, MW (2 g: 60 mL, 2 min heating (50 °C/min) + 5.5 min (120 °C) |
| E4 | SCG (Dry 105 °C/ 8h), MW (2 g: 60 mL, 2 min heating (90 °C/min) + 2 min (200 °C) | |
| E5 | IC, Solid/liquid extraction (15 g: 500 mL, 80 °C, 10 min) | |
| Sample Designation | Sugar Composition (% mol) | Total Sugars (gsugar/gsample) | Polysaccharides Content (gpolysaccharide/gsample) | Ratio | |||||
|---|---|---|---|---|---|---|---|---|---|
| Coffee Samples | Rha | Ara | Man | Gal | Glc | AGa) | GMb) | AG/GM | |
| S1 | 6.2 | 19.5 | 22.1 | 45.5 | 6.6 | 0.25 | 0.16 | 0.06 | 2.6 |
| S2 | 3.3 | 13.5 | 45.6 | 30.8 | 6.9 | 0.26 | 0.1 | 0.13 | 0.8 |
| S3 | 5.3 | 16.9 | 35.8 | 29.6 | 12.4 | 0.24 | 0.11 | 0.1 | 1.1 |
| Coffee Extracts | |||||||||
| E1 | 5.5 | 20.5 | 33.3 | 34.4 | 6.3 | 0.16 | 0.08 | 0.06 | 1.4 |
| E2 | 3.7 | 20.1 | 41.4 | 29.7 | 5 | 0.22 | 0.1 | 0.1 | 1 |
| E3 | 3.4 | 12.4 | 55.1 | 25.7 | 3.4 | 0.26 | 0.09 | 0.15 | 0.6 |
| E4 | 0.0 | 12.6 | 18.7 | 66.8 | 1.2 | 0.67 | 0.52 | 0.14 | 3.8 |
| E5 | 2.4 | 8.5 | 13.4 | 73.9 | 0.8 | 0.52 | 0.42 | 0.08 | 5.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coreta-Gomes, F.M.; Lopes, G.R.; Passos, C.P.; Vaz, I.M.; Machado, F.; Geraldes, C.F.G.C.; Moreno, M.J.; Nyström, L.; Coimbra, M.A. In Vitro Hypocholesterolemic Effect of Coffee Compounds. Nutrients 2020, 12, 437. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12020437
Coreta-Gomes FM, Lopes GR, Passos CP, Vaz IM, Machado F, Geraldes CFGC, Moreno MJ, Nyström L, Coimbra MA. In Vitro Hypocholesterolemic Effect of Coffee Compounds. Nutrients. 2020; 12(2):437. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12020437
Chicago/Turabian StyleCoreta-Gomes, Filipe Manuel, Guido R. Lopes, Cláudia P. Passos, Inês M. Vaz, Fernanda Machado, Carlos F. G. C. Geraldes, Maria João Moreno, Laura Nyström, and Manuel A. Coimbra. 2020. "In Vitro Hypocholesterolemic Effect of Coffee Compounds" Nutrients 12, no. 2: 437. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12020437