Vitamin D and Endothelial Function
Abstract
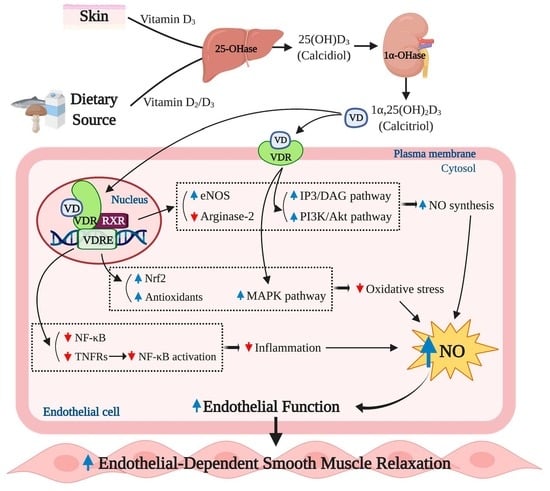
:1. Introduction
2. Basic Physiology of Vitamin D and the Endothelium
3. Effect of Vitamin D on Regulation of Nitric Oxide (NO) Bioavailability
4. Anti-Oxidant Capacity of Vitamin D Reduces Oxidative Stress-Induced Endothelial Dysfunction
5. Anti-Inflammatory Effects of Vitamin D and Endothelial Function
6. Review of Clinical Trials: The Effect of Vitamin D Supplementation on Endothelial Function
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fleming, I.; Busse, R. Signal transduction of eNOS activation. Cardiovasc. Res. 1999, 43, 532–541. [Google Scholar] [CrossRef] [Green Version]
- Dedeoglu, M.; Garip, Y.; Bodur, H. Osteomalacia in Crohn’s disease. Arch. Osteoporos. 2014. [Google Scholar] [CrossRef] [PubMed]
- Kanikarla-Marie, P.; Jain, S.K. 1,25(OH) 2 D 3 inhibits oxidative stress and monocyte adhesion by mediating the upregulation of GCLC and GSH in endothelial cells treated with acetoacetate (ketosis). J. Steroid Biochem. Mol. Biol. 2016. [Google Scholar] [CrossRef] [Green Version]
- Bischoff, H.; Borchers, M.; Gudat, F.; Duermueller, U.; Theiler, R.; Stähelin, H.; Dick, W. In Situ Detection of 1,25-dihydroxyvitamin D Receptor In human Skeletal Muscle Tissue. Histochem. J. 2001, 33, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; De Nigris, F.; Williams-Ignarro, S.; Pignalosa, O.; Sica, V.; Ignarro, L.J. Nitric oxide and atherosclerosis: An update. Nitric Oxide Biol. Chem. 2006, 15, 265–279. [Google Scholar] [CrossRef]
- González-Molero, I.; Rojo-Martínez, G.; Morcillo, S.; Gutiérrez, C.; Rubio-Martín, E.; Pérez-Valero, V.; Esteva, I.; De Adana, M.S.R.; Almaraz, M.C.; Colomo, N.; et al. Hypovitaminosis D and incidence of obesity: A prospective study. Eur. J. Clin. Nutr. 2013, 67, 680–682. [Google Scholar] [CrossRef] [Green Version]
- Sokol, S.I.; Srinivas, V.; Crandall, J.P.; Kim, M.; Tellides, G.; Lebastchi, A.; Yu, Y.; Gupta, A.K.; Alderman, M.H. The effects of vitamin D repletion on endothelial function and inflammation in patients with coronary artery disease. Vasc. Med. 2012. [Google Scholar] [CrossRef] [Green Version]
- Labudzynskyi, D.O.; Zaitseva, O.V.; Latyshko, N.V.; Gud Kova, O.O.; Veliky, M.M. Vitamin D3 contribution to the regulation of oxidative metabolism in the liver of diabetic mice. Ukr. Biochem. J. 2015, 87, 75–90. [Google Scholar] [CrossRef] [Green Version]
- Barthelmes, J.; Nägele, M.P.; Ludovici, V.; Ruschitzka, F.; Sudano, I.; Flammer, A.J. Endothelial dysfunction in cardiovascular disease and Flammer syndrome-similarities and differences. EPMA J. 2017, 8, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.-Y.; Jiang, C.; Sun, C.; Tang, T.-F.; Jin, B.; Cao, D.-W.; He, J.; Zhang, M. Hypovitaminosis D is associated with endothelial dysfunction in patients with non-dialysis chronic kidney disease. J. Nephrol. 2014, 28, 471–476. [Google Scholar] [CrossRef]
- Equils, O.; Naiki, Y.; Shapiro, A.M.; Michelsen, K.; Lu, D.; Adams, J.; Jordan, S. 1,25-Dihydroxyvitamin D 3 inhibits lipopolysaccharide-induced immune activation in human endothelial cells. Clin. Exp. Immunol. 2006. [Google Scholar] [CrossRef] [PubMed]
- Zella, J.B.; DeLuca, H.F. Vitamin D and autoimmune diabetes. J. Cell. Biochem. 2003, 88, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D and bone health. J. Nutr. 1996, 126, 1159S–1164S. [Google Scholar] [CrossRef] [PubMed]
- Deluca, H.F.; Cantorna, M.T. Vitamin D: Its role and uses in immunology. FASEB J. 2001, 15, 2579–2585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dulak, J.; Józkowicz, A.; Dembinska-Kiec, A.; Guevara, I.; Zdzienicka, A.; Zmudzinska-Grochot, D.; Florek, I.; Wójtowicz, A.; Szuba, A.; Cooke, J.P. Nitric oxide induces the synthesis of vascular endothelial growth factor by rat vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2000. [CrossRef] [Green Version]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; Van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; DeMay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef]
- Radomski, M.W.; Palmer, R.M.J.; Moncada, S. The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochem. Biophys. Res. Commun. 1987. [Google Scholar] [CrossRef]
- Ramagopalan, S.V.; Heger, A.; Berlanga, A.J.; Maugeri, N.J.; Lincoln, M.R.; Burrell, A.; Handunnetthi, L.; Handel, A.; Disanto, G.; Orton, S.-M.; et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: Associations with disease and evolution. Genome Res. 2010, 20, 1352–1360. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.J.; Forman, H.J.; Sevanian, A. Oxidants as stimulators of signal transduction. Free Radic. Biol. Med. 1997. [Google Scholar] [CrossRef]
- Haussler, M.R.; Haussler, C.A.; Jurutka, P.W.; Thompson, P.D.; Hsieh, J.C.; Remus, L.S.; Selznick, S.H.; Whitfield, G.K. The vitamin D hormone and its nuclear receptor: Molecular actions and disease states. J. Endocrinol. 1997, 154 (Suppl.), S57–S73. [Google Scholar]
- Holick, M.F. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am. J. Clin. Nutr. 2004, 79, 362–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakshmi, S.V.V.; Padmaja, G.; Kuppusamy, P.; Kutala, V.K. Oxidative stress in cardiovascular disease. Indian J. Biochem. Biophys. 2009, 46, 421–440. [Google Scholar] [PubMed]
- Hingorani, A.D. Polymorphisms in endothelial nitric oxide synthase and atherogenesis: John French Lecture 2000. Atherosclerosis 2001, 154, 521–527. [Google Scholar] [CrossRef]
- Donato, A.J.; Pierce, G.L.; Lesniewski, L.A.; Seals, D.R. Role of NFkappaB in age-related vascular endothelial dysfunction in humans. Aging 2009, 1, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Feghali, C.A.; Wright, T.M. Cytokines in acute and chronic inflammation. Front. Biosci. 1997, 2, d12–d16. [Google Scholar] [PubMed] [Green Version]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.C.; Jurutka, P.W. Molecular mechanisms of vitamin D action. Calcif. Tissue Int. 2013. [Google Scholar] [CrossRef]
- Forrest, K.Y.Z.; Stuhldreher, W.L. Prevalence and correlates of vitamin D deficiency in US adults. Nutr. Res. 2011. [Google Scholar] [CrossRef]
- Dharmashankar, K.; Widlansky, M.E. Vascular endothelial function and hypertension: Insights and directions. Curr. Hypertens. Rep. 2010, 12, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Guzik, T.J.; Harrison, D.G. Endothelial NF-κB as a mediator of kidney damage: The missing link between systemic vascular and renal disease? Circ. Res. 2007, 101, 227–229. [Google Scholar] [CrossRef] [Green Version]
- Hussin, A.M.; Ashor, A.W.; Schoenmakers, I.; Hill, T.; Mathers, J.C.; Siervo, M. Effects of vitamin D supplementation on endothelial function: A systematic review and meta-analysis of randomised clinical trials. Eur. J. Nutr. 2017, 56, 1095–1104. [Google Scholar] [CrossRef]
- Higashi, Y.; Noma, K.; Yoshizumi, M.; Kihara, Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ. J. 2009, 73, 411–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadeghi, K.; Wessner, B.; Laggner, U.; Ploder, M.; Tamandl, D.; Friedl, J.; Zügel, U.; Steinmeyer, A.; Pollak, A.; Roth, E.; et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur. J. Immunol. 2006, 36, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, G.; de Boland, A.R.; Boland, R. Stimulation of Ca2+Release-Activated Ca2+Channels as a Potential Mechanism Involved in Non-Genomic 1,25(OH)2-Vitamin D3-Induced Ca2+Entry in Skeletal Muscle Cells. Biochem. Biophys. Res. Commun. 1997, 239, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Cayatte, A.J.; Palacino, J.J.; Horten, K.; Cohen, R.A. Chronic inhibition of nitric oxide production accelerates neointima formation and impairs endothelial function in hypercholesterolemic rabbits. Arterioscler. Thromb. Vasc. Biol. 1994. [Google Scholar] [CrossRef] [PubMed]
- Oldham, K.M.; Bowen, P.E. Oxidative stress in critical care: Is antioxidant supplementation beneficial? J. Am. Diet. Assoc. 1998, 98, 1001–1008. [Google Scholar] [CrossRef]
- Igari, K.; Kudo, T.; Toyofuku, T.; Inoue, Y. Endothelial Dysfunction of Patients with Peripheral Arterial Disease Measured by Peripheral Arterial Tonometry. Int. J. Vasc. Med. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ignarro, L. The Pharmacological and Physiological Role of Cyclic GMP in Vascular Smooth Muscle Relaxation. Annu. Rev. Pharmacol. Toxicol. 1985. [Google Scholar] [CrossRef]
- Tohari, A.M.; Alhasani, R.H.; Biswas, L.; Patnaik, S.R.; Reilly, J.; Zeng, Z.; Shu, X. Vitamin D attenuates oxidative damage and inflammation in retinal pigment epithelial cells. Antioxidants 2019, 8, 341. [Google Scholar] [CrossRef] [Green Version]
- Abdali, D.; Samson, S.E.; Grover, A.K. How effective are antioxidant supplements in obesity and diabetes? Med. Princ. Pract. 2015, 24, 201–215. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980. [Google Scholar] [CrossRef]
- Polidoro, L.; Properzi, G.; Marampon, F.; Gravina, G.L.; Festuccia, C.; Di Cesare, E.; Scarsella, L.; Ciccarelli, C.; Zani, B.M.; Ferri, C. Vitamin D protects human endothelial cells from H2O2 oxidant injury through the Mek/Erk-sirt1 axis activation. J. Cardiovasc. Transl. Res. 2013. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Fujii, H.; Kono, K.; Goto, S.; Kitazawa, R.; Kitazawa, S.; Hirata, M.; Shinohara, M.; Fukagawa, M.; Nishi, S. Vitamin D activates the Nrf2-keap1 antioxidant pathway and ameliorates nephropathy in diabetic rats. Am. J. Hypertens. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandenburg, V.M.; Vervloet, M.G.; Marx, N. The role of vitamin D in cardiovascular disease: From present evidence to future perspectives. Atherosclerosis 2012, 225, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Andrukhova, O.; Slavic, S.; Zeitz, U.; Riesen, S.C.; Heppelmann, M.S.; Ambrisko, T.D.; Markovic, M.; Kuebler, W.M.; Erben, R.G. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Mol. Endocrinol. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.C.; Pirro, A.E.; Amling, M.; Delling, G.; Baron, R.; Bronson, R.; Demay, M.B. Targeted ablation of the vitamin D receptor: An animal model of vitamin D-dependent rickets type II with alopecia. Proc. Natl. Acad. Sci. USA 1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleet, J.C. Rapid, Membrane-Initiated Actions of 1,25 Dihydroxyvitamin D: What Are They and What Do They Mean? J. Nutr. 2004. [Google Scholar] [CrossRef] [PubMed]
- Busse, R.; Fleming, I. Regulation and functional consequences of endothelial nitric oxide formation. Ann. Med. 1995. [Google Scholar] [CrossRef]
- Schiffrin, E.L. A critical review of the role of endothelial factors in the pathogenesis of hypertension. J. Cardiovasc. Pharmacol. 2002, 38, S3–S5. [Google Scholar] [CrossRef] [Green Version]
- Umans, J. Nitric Oxide in the Regulation of Blood Flow and Arterial Pressure. Annu. Rev. Physiol. 1995. [Google Scholar] [CrossRef]
- Vazquez, G.; De Boland, A.R. Involvement of protein kinase C in the modulation of 1α,25-dihydroxy-vitainin D3-induced 45Ca2+ uptake in rat and chick cultured myoblasts. Biochim. Biophys. Acta Mol. Cell Res. 1996, 1310, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Busse, R.; Mülsch, A. Calcium-dependent nitric oxide synthesis in endothelial cytosol is mediated by calmodulin. FEBS Lett. 1990. [Google Scholar] [CrossRef] [Green Version]
- Buitrago, C.; Pardo, V.G.; Boland, R. Role of VDR in 1α,25-dihydroxyvitamin D3-dependent non-genomic activation of MAPKs, Src and Akt in skeletal muscle cells. J. Steroid Biochem. Mol. Biol. 2013, 136, 125–130. [Google Scholar] [CrossRef]
- Molinari, C.; Rizzi, M.; Squarzanti, D.F.; Pittarella, P.; Vacca, G.; Renò, F. 1α,25-dihydroxycholecalciferol (vitamin D3) induces NO-dependent endothelial cell proliferation and migration in a three-dimensional matrix. Cell. Physiol. Biochem. 2013, 31, 815–822. [Google Scholar] [CrossRef]
- Mazidi, M.; Karimi, E.; Rezaie, P.; Vatanparast, H. The impact of vitamin D supplement intake on vascular endothelial function; a systematic review and meta-analysis of randomized controlled trials. Food Nutr. Res. 2016, 61, 1273574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griendling, K.K.; Sorescu, D.; Lassègue, B.; Ushio-Fukai, M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2175–2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michell, B.J.; Griffiths, J.E.; Mitchelhill, K.I.; Rodriguez-Crespo, I.; Tiganis, T.; Bozinovski, S.; de Montellano, P.R.O.; Kemp, B.E.; Pearson, R.B. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr. Biol. 1999. [Google Scholar] [CrossRef] [Green Version]
- Pietsch, E.C.; Chan, J.Y.; Torti, F.M.; Torti, S.V. Nrf2 mediates the induction of ferritin H in response to xenobiotics and cancer chemopreventive dithiolethiones. J. Biol. Chem. 2003. [Google Scholar] [CrossRef] [Green Version]
- Babaei, S.; Teichert-Kuliszewska, K.; Monge, J.C.; Mohamed, F.; Bendeck, M.P.; Stewart, D.J. Role of nitric oxide in the angiogenic response in vitro to basic fibroblast growth factor. Circ. Res. 1998. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H.; Henry, P.D. Atherosclerosis as a microvascular disease: Impaired angiogenesis mediated by suppressed basic fibroblast growth factor expression. Proc. Assoc. Am. Physicians 1997, 109, 351–361. [Google Scholar]
- Palmer, R.M.J.; Ashton, D.S.; Moncada, S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature 1988. [Google Scholar] [CrossRef]
- Ziche, M.; Morbidelli, L.; Masini, E.; Amerini, S.; Granger, H.J.; Maggi, C.A.; Geppetti, P.; Ledda, F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J. Clin. Investig. 1994. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Doroudi, M.; Schwartz, Z.; Boyan, B.D. Membrane-mediated actions of 1,25-dihydroxy vitamin D3: A review of the roles of phospholipase A2 activating protein and Ca2+/calmodulin-dependent protein kinase II. J. Steroid Biochem. Mol. Biol. 2015, 147, 81–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundwall, K.; Jacobson, S.H.; Jörneskog, G.; Spaak, J. Treating endothelial dysfunction with vitamin D in chronic kidney disease: A meta-analysis. BMC Nephrol. 2018, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ziche, M.; Parenti, A.; Ledda, F.; DelL’Era, P.; Granger, H.J.; Maggi, C.A.; Presta, M. Nitric oxide promotes proliferation and plasminogen activator production by coronary venular endothelium through endogenous bFGF. Circ. Res. 1997. [Google Scholar] [CrossRef] [PubMed]
- Meier, B.; Radeke, H.H.; Selle, S.; Younes, M.; Sies, H.; Resch, K.; Habermehl, G.G. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-α. Biochem. J. 1989. [Google Scholar] [CrossRef]
- Hernández-Presa, M.; Bustos, C.; Ortego, M.; Tuñon, J.; Renedo, G.; Ruiz-Ortega, M.; Egido, J. Angiotensin-converting enzyme inhibition prevents arterial nuclear factor-κB activation, monocyte chemoattractant protein-1 expression, and macrophage infiltration in a rabbit model of early accelerated atherosclerosis. Circulation 1997. [Google Scholar] [CrossRef]
- Maulik, N.; Das, D.K. Redox signaling in vascular angiogenesis. Free Radic. Biol. Med. 2002. [Google Scholar] [CrossRef]
- Ning, C.; Liu, L.; Lv, G.; Yang, Y.; Zhang, Y.; Yu, R.; Wang, Y.; Zhu, J. Lipid metabolism and inflammation modulated by Vitamin D in liver of diabetic rats. Lipids Health Dis. 2015, 14, 31. [Google Scholar] [CrossRef] [Green Version]
- Kwak, M.K.; Egner, P.A.; Dolan, P.M.; Ramos-Gomez, M.; Groopman, J.D.; Itoh, K.; Yamamoto, M.; Kensler, T.W. Role of phase 2 enzyme induction in chemoprotection by dithiolethiones. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2001. [Google Scholar] [CrossRef]
- Grandi, N.C.; Breitling, L.P.; Brenner, H. Vitamin D and cardiovascular disease: Systematic review and meta-analysis of prospective studies. Prev. Med. 2010, 51, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Miguel, P.; Valdivielso, J.M.; Medrano-Andrés, D.; Román-García, P.; Cano-Peñalver, J.L.; Rodríguez-Puyol, M.; Rodríguez-Puyol, D.; López-Ongil, S. The active form of vitamin D, calcitriol, induces a complex dual upregulation of endothelin and nitric oxide in cultured endothelial cells. Am. J. Physiol. Endocrinol. Metab. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, Y.; Ichiyama, T.; Ohsaki, A.; Hasegawa, S.; Shiraishi, M.; Furukawa, S. Anti-inflammatory effect of 1α,25-dihydroxyvitamin D3 in human coronary arterial endothelial cells: Implication for the treatment of Kawasaki disease. J. Steroid Biochem. Mol. Biol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Christopher Sill, J.; Katušić, Z.S. Role of superoxide anions in the mediation of endothelium-dependent contractions. Hypertension 1994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendrick, J.; Andrews, E.; You, Z.; Moreau, K.; Nowak, K.L.; Farmer-Bailey, H.; Seals, D.R.; Chonchol, M. Cholecalciferol, calcitriol, and vascular function in CKD a randomized, double-blind trial. Clin. J. Am. Soc. Nephrol. 2017. [Google Scholar] [CrossRef] [Green Version]
- Zalba, G.; José, G.S.; Moreno, M.U.; Fortuño, M.A.; Fortuño, A.; Beaumont, F.J.; Díez, J. Oxidative stress in arterial hypertension role of NAD(P)H oxidase. Hypertension. 2001, 38, 1395–1399. [Google Scholar] [CrossRef]
- Verma, S.; Anderson, T.J. Fundamentals of Endothelial Function for the Clinical Cardiologist. Circulation 2002, 105, 546–549. [Google Scholar] [CrossRef] [Green Version]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and the ugly. Am. J. Physiol. Cell Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khafaji, H.A.H.; Al Suwaidi, J. Endothelial dysfunction in diabetes mellitus. Vasc. Heal. Risk Manag. 2007, 3, 853–876. [Google Scholar]
- Zou, M.-H.; Shi, C.; Cohen, R.A. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J. Clin. Investig. 2013, 109, 817–826. [Google Scholar] [CrossRef]
- Hewitt, N.A.; O’Connor, A.A.; O’Shaughnessy, D.V.; Elder, G.J. Effects of cholecalciferol on functional, biochemical, vascular, and quality of life outcomes in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2013, 8, 1143–1149. [Google Scholar] [CrossRef] [Green Version]
- Codoñer-Franch, P.; Tavárez-Alonso, S.; Simó-Jordá, R.; Laporta-Martín, P.; Carratalá-Calvo, A.; Alonso-Iglesias, E. Vitamin D status is linked to biomarkers of oxidative stress, inflammation, and endothelial activation in obese children. J. Pediatr. 2012, 161, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Victor, V.M.; Rocha, M.; Solá, E.; Bañuls, C.; Garcia-Malpartida, K.; Hernandez-Mijares, A. Oxidative stress, endothelial dysfunction and atherosclerosis. Curr. Pharm. Des. 2009, 15, 2988–3002. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Masutani, H.; Yamaguchi, Y.; Itoh, K.; Yamamoto, M.; Yodoi, J. Hemin-induced activation of the thioredoxin gene by Nrf2: A differential regulation of the antioxidant responsive element by a switch of its binding factors. J. Biol. Chem. 2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kono, K.; Fujii, H.; Nakai, K.; Goto, S.; Kitazawa, R.; Kitazawa, S.; Shinohara, M.; Hirata, M.; Fukagawa, M.; Nishi, S. Anti-oxidative effect of vitamin d analog on incipient vascular lesion in non-obese type 2 diabetic rats. Am. J. Nephrol. 2013, 37, 167–174. [Google Scholar] [CrossRef]
- Pittarella, P.; Squarzanti, D.F.; Molinari, C.; Invernizzi, M.; Uberti, F.; Renò, F. NO-dependent proliferation and migration induced by Vitamin D in HUVEC. J. Steroid Biochem. Mol. Biol. 2015, 149, 35–42. [Google Scholar] [CrossRef]
- Jain, S.K.; Micinski, D.; Huning, L.; Kahlon, G.; Bass, P.F.; Levine, S.N. Vitamin D and L-cysteine levels correlate positively with GSH and negatively with insulin resistance levels in the blood of type 2 diabetic patients. Eur. J. Clin. Nutr. 2014. [Google Scholar] [CrossRef] [Green Version]
- Jablonski, K.L.; Chonchol, M.; Pierce, G.L.; Walker, A.E.; Seals, D.R. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension 2011, 57, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Montenegro, K.R.; Cruzat, V.; Carlessi, R.; Newsholme, P. Mechanisms of vitamin D action in skeletal muscle. Nutr. Res. Rev. 2019. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.; Kan, Y.W. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 12731–12736. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.Y.; Jedlicka, A.E.; Reddy, S.P.M.; Kensler, T.W.; Yamamoto, M.; Zhang, L.Y.; Kleeberger, S.R. Role of NRF2 in protection against hyperoxic lung injury in mice. Am. J. Respir. Cell Mol. Biol. 2002, 26, 175–182. [Google Scholar] [CrossRef]
- Ignarro, L.J. Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension 1990, 16, 477–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerr, S.; Brosnan, M.J.; McIntyre, M.; Reid, J.L.; Dominiczak, A.F.; Hamilton, C.A. Superoxide anion production is increased in a model of genetic hypertension: Role of the endothelium. Hypertension 1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kissner, R.; Nauser, T.; Bugnon, P.; Lye, P.G.; Koppenol, W.H. Formation and properties of peroxynitrite as studied by laser flash photolysis, high-pressure stopped-flow technique, pulse radiolysis. Chem. Res. Toxicol. 1997. [Google Scholar] [CrossRef]
- Pierce, G.L.; Lesniewski, L.A.; Lawson, B.R.; Beske, S.D.; Seals, D.R. Nuclear factor-κB activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesarik, J.; Mendoza, C. Direct non-genomic effects of follicular steroids on maturing human oocytes: Oestrogen versus androgen antagonism. Hum. Reprod. Update 1997, 3, 95–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zhuang, X.-D.; Meng, F.-H.; Chen, L.; Dong, X.-B.; Liu, G.-H.; Li, J.-H.; Dong, Q.; Xu, J.-D.; Yang, C.-T. Calcitriol prevents peripheral RSC96 Schwann neural cells from high glucose & methylglyoxal-induced injury through restoration of CBS/H 2 S expression. Neurochem. Int. 2016, 92, 49–57. [Google Scholar]
- Antuna-Puente, B.; Feve, B.; Fellahi, S.; Bastard, J.-P. Adipokines: The missing link between insulin resistance and obesity. Diabetes Metab. 2008, 34, 2–11. [Google Scholar] [CrossRef]
- Ertek, S.; Akgül, E.; Cicero, A.F.; Kütük, U.; Demirtaş, S.; Çehreli, S.; Erdoǧan, G. 25-hydroxy vitamin D levels and endothelial vasodilator function in normotensive women. Arch. Med. Sci. 2012. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.; Pittas, A.G.; Del Gobbo, L.C.; Zhang, C.; Manson, J.E.; Hu, F.B. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care 2013, 36, 1422–1428. [Google Scholar] [CrossRef] [Green Version]
- Brezinski, E.; Follansbee, M.; Armstrong, E.; Armstrong, A. Endothelial Dysfunction and the Effects of TNF Inhibitors on the Endothelium in Psoriasis and Psoriatic Arthritis: A Systematic Review. Curr. Pharm. Des. 2014. [Google Scholar] [CrossRef] [Green Version]
- Bergholm, R.; Leirisalo-Repo, M.; Vehkavaara, S.; Mäkimattila, S.; Taskinen, M.R.; Yki-Järvinen, H. Impaired responsiveness to NO in newly diagnosed patients with rheumatoid arthritis. Arterioscler. Thromb. Vasc. Biol. 2002. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Itoh, K.; Takahashi, S.; Sato, H.; Yanagawa, T.; Katoh, Y.; Bannai, S.; Yamamoto, M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csiszar, A.; Wang, M.; Lakatta, E.G.; Ungvari, Z. Inflammation and endothelial dysfunction during aging: Role of NF-κB. J. Appl. Physiol. 2008, 105, 1333–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Winther, M.P.J.; Kanters, E.; Kraal, G.; Hofker, M.H. Nuclear factor κB signaling in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 904–914. [Google Scholar] [CrossRef] [Green Version]
- Papapetropoulos, A.; García-Cardeña, G.; Madri, J.A.; Sessa, W.C. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J. Clin. Investig. 1997. [Google Scholar] [CrossRef]
- Donato, A.J.; Black, A.D.; Jablonski, K.L.; Gano, L.B.; Seals, D.R. Aging is associated with greater nuclear NFκB, reduced IκBα, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell 2008, 7, 805–812. [Google Scholar] [CrossRef] [Green Version]
- Grundmann, M.; Haidar, M.; Placzko, S.; Niendorf, R.; Darashchonak, N.; Hubel, C.A.; Von Versen-Höynck, F. Vitamin D improves the angiogenic properties of endothelial progenitor cells. Am. J. Physiol. Cell Physiol. 2012. [Google Scholar] [CrossRef]
- Wang, G.P.; Deng, Z.D.; Ni, J.; Qu, Z.L. Oxidized low density lipoprotein and very low density lipoprotein enhance expression of monocyte chemoattractant protein-1 in rabbit peritoneal exudate macrophages. Atherosclerosis 1997. [Google Scholar] [CrossRef]
- Zhang, C.; Hein, T.W.; Wang, W.; Ren, Y.; Shipley, R.D.; Kuo, L. Activation of JNK and xanthine oxidase by TNF-α impairs nitric oxide-mediated dilation of coronary arterioles. J. Mol. Cell. Cardiol. 2006. [Google Scholar] [CrossRef]
- Yang, J.; Park, Y.; Zhang, H.; Gao, X.; Wilson, E.; Zimmer, W.; Abbott, L.; Zhang, C. Role of MCP-1 in tumor necrosis factor-α-induced endothelial dysfunction in type 2 diabetic mice. Am. J. Physiol. Hear. Circ. Physiol. 2009. [Google Scholar] [CrossRef] [Green Version]
- Wang, E.W.; Siu, P.; Pang, M.Y.C.; Woo, J.; Collins, A.R.; Benzie, I. Vitamin D deficiency, oxidative stress and antioxidant status: Only weak association seen in the absence of advanced age, obesity or pre-existing disease. Br. J. Nutr. 2017, 118, 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giulietti, A.; Van Etten, E.; Overbergh, L.; Stoffels, K.; Bouillon, R.; Mathieu, C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D3 works as anti-inflammatory. Diabetes Res. Clin. Pract. 2007, 77, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Leung, N.Y.M.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef] [Green Version]
- Ebihara, K.; Masuhiro, Y.; Kitamoto, T.; Suzawa, M.; Uematsu, Y.; Yoshizawa, T.; Ono, T.; Harada, H.; Matsuda, K.; Hasegawa, T.; et al. Intron retention generates a novel isoform of the murine vitamin D receptor that acts in a dominant negative way on the vitamin D signaling pathway. Mol. Cell. Biol. 1996, 16, 3393–3400. [Google Scholar] [CrossRef] [Green Version]
- Sugden, J.A.; Davies, J.I.; Witham, M.D.; Morris, A.D.; Struthers, A.D. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet. Med. 2008. [Google Scholar] [CrossRef]
- Cohen-Lahav, M.; Shany, S.; Tobvin, D.; Chaimovitz, C.; Douvdevani, A. Vitamin D decreases NFkappaB activity by increasing IkappaBalpha levels. Nephrol. Dial. Transpl. 2006, 21, 889–897. [Google Scholar] [CrossRef] [Green Version]
- Revelli, A.; Massobrio, M.; Tesarik, J. Nongenomic actions of steroid hormones in reproductive tissues. Endocr. Rev. 1998, 19, 3–17. [Google Scholar]
- Gepner, A.D.; Ramamurthy, R.; Krueger, D.C.; Korcarz, C.E.; Binkley, N.; Stein, J.H. A prospective randomized controlled trial of the effects of Vitamin D supplementation on cardiovascular disease risk. PLoS ONE 2012, 7, e36617. [Google Scholar] [CrossRef] [Green Version]
- Ni, W.; Watts, S.W.; Ng, M.; Chen, S.; Glenn, D.J.; Gardner, D.G. Elimination of vitamin D receptor in vascular endothelial cells alters vascular function. Hypertension 2014. [Google Scholar] [CrossRef] [Green Version]
- Breslavsky, A.; Frand, J.; Matas, Z.; Boaz, M.; Barnea, Z.; Shargorodsky, M. Effect of high doses of vitamin D on arterial properties, adiponectin, leptin and glucose homeostasis in type 2 diabetic patients. Clin. Nutr. 2013, 32, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Grundy, S.M.; Unger, R.H. Comparison of effects of high and low carbohydrate diets on plasma lipoproteins and insulin sensitivity in patients with mild NIDDM. Diabetes 1992, 41, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, Z.; Shaked, D.; Hardin, R.R.; Gruwell, S.; Dean, D.D.; Sylvia, V.L.; Boyan, B.D. 1α,25(OH)2D3 causes a rapid increase in phosphatidylinositol-specific PLC-β activity via phospholipase A2-dependent production of lysophospholipid. Steroids 2003. [Google Scholar] [CrossRef]
- Steyers, C.M.; Miller, F.J. Endothelial dysfunction in chronic inflammatory diseases. Int. J. Mol. Sci. 2014, 15, 11324–11349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witham, M.D.; Dove, F.J.; Khan, F.; Lang, C.C.; Belch, J.J.F.; Struthers, A.D. Effects of Vitamin D supplementation on markers of vascular function after myocardial infarction—A randomised controlled trial. Int. J. Cardiol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Nezhad, A.; Mirzaei, K.; Keshavarz, S.A.; Ansar, H.; Saboori, S.; Tootee, A. Evidences of dual role of vitamin D through cellular energy homeostasis and inflammation pathway in risk of cancer in obese subjects. Minerva Med. 2013, 104, 295–307. [Google Scholar]
- Lerman, A.; Burnett, J.C. Intact and altered endothelium in regulation of vasomotion. Circulation 1992, 86 (Suppl. 6), III12-19. [Google Scholar]
- Matsunaga, T.; Weihrauch, D.W.; Moniz, M.C.; Tessmer, J.; Warltier, D.C.; Chilian, W.M. Angiostatin inhibits coronary angiogenesis during impaired production of nitric oxide. Circulation 2002. [Google Scholar] [CrossRef] [Green Version]
- Stricker, H.; Tosi Bianda, F.; Guidicelli-Nicolosi, S.; Limoni, C.; Colucci, G. Effect of a single, oral, high-dose vitamin D supplementation on endothelial function in patients with peripheral arterial disease: A randomised controlled pilot study. Eur. J. Vasc. Endovasc. Surg. 2012. [Google Scholar] [CrossRef] [Green Version]
- Chitalia, N.; Ismail, T.; Tooth, L.; Boa, F.; Hampson, G.; Goldsmith, D.; Kaski, J.C.; Banerjee, D. Impact of vitamin D supplementation on arterial vasomotion, stiffness and endothelial biomarkers in chronic kidney disease patients. PLoS ONE 2014, 9, e91363. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zhang, M.; Wang, H.; Sun, C.; Feng, Y.; Zhu, W.; Cao, D.; Shao, Q.; Li, N.; Xia, Y.; et al. Vitamin D supplementation improves endothelial dysfunction in patients with non-dialysis chronic kidney disease. Int. Urol. Nephrol. 2018, 50, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Kato, S. The function of vitamin D receptor in vitamin D action. J. Biochem. 2000. [CrossRef] [PubMed]
- Yiu, Y.F.; Yiu, K.H.; Siu, C.W.; Chan, Y.H.; Li, S.W.; Wong, L.Y.; Lee, S.W.L.; Tam, S.; Wong, E.W.K.; Lau, C.P.; et al. Randomized controlled trial of vitamin D supplement on endothelial function in patients with type 2 diabetes. Atherosclerosis 2013. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-H.; Meza, C.A.; Clarke, H.; Kim, J.-S.; Hickner, R.C. Vitamin D and Endothelial Function. Nutrients 2020, 12, 575. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12020575
Kim D-H, Meza CA, Clarke H, Kim J-S, Hickner RC. Vitamin D and Endothelial Function. Nutrients. 2020; 12(2):575. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12020575
Chicago/Turabian StyleKim, Do-Houn, Cesar A. Meza, Holly Clarke, Jeong-Su Kim, and Robert C. Hickner. 2020. "Vitamin D and Endothelial Function" Nutrients 12, no. 2: 575. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12020575