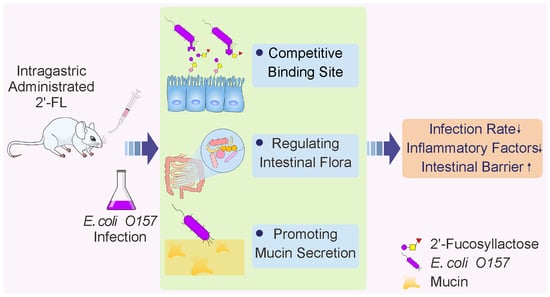
The Protective Effects of 2’-Fucosyllactose Against E. Coli O157 Infection Are Mediated by the Regulation of Gut Microbiota and the Inhibition of Pathogen Adhesion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Determination of Infection in Mice
2.3. Preparation of Pathological Sections
2.4. Analysis of Inflammatory Levels in Serum and Intestine of the Mice
2.5. Analysis of mRNA Expression of Mucin- and Occludin-Related Genes
2.6. 16S rDNA Gene Sequencing of the Cecum of the Mice
2.7. Short-Chain Fatty Acid Quantification
2.8. Cell Culture and Adhesion Experiments
2.9. Statistical Analysis
3. Results
3.1. 2’-FL inhibit Colonization of E. Coli O157 in Intestine
3.2. 2’-FL Lowers Inflammatory Levels and the mRNA Expression of Mucin- and Occludin-Related Genes
3.3. Effects of 2’-FL on Gut Microbiota Composition
3.4. 2’-FL Increases Content of SCFAs in Colon
3.5. 2’-FL Inhibits Adhesion of E. Coli O157 to Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stephan, T.; Manfred, M.; Günther, B.; Catherine, M.; Bernd, S. Systematic review of the concentrations of oligosaccharides in human milk. Nutr. Rev. 2017, 75, 920–933. [Google Scholar]
- Bych, K.; Mikš, M.H.; Johanson, T.; Hederos, M.J.; Vigsnæs, L.K.; Becker, P. Production of HMOs using microbial hosts—From cell engineering to large scale production. Curr. Opin. Biotechnol. 2019, 56, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Wang, J.; Hang, Y.; Zhang, Y.; Yu, H.; Li, Z.; Pan, L.; Dai, Z. A human milk oligosaccharide, 2’-fucosyllactose, enhances the immunity in mice fed an infant formula milk diet. Int. Dairy J. 2019, 98, 38–43. [Google Scholar] [CrossRef]
- Grabinger, T.; Garzon, J.F.G.; Hausmann, M.; Geirnaert, A.; Lacroix, C.; Hennet, T. Alleviation of Intestinal Inflammation by Oral Supplementation with 2-Fucosyllactose in Mice. Front. Microbiol. 2019, 10, 1385. [Google Scholar] [CrossRef]
- Oliveros, E.; Ramirez, M.; Vazquez, E.; Barranco, A.; Gruart, A.; Maria Delgado-Garcia, J.; Buck, R.; Rueda, R.; Martin, M.J. Oral supplementation of 2’-fucosyllactose during lactation improves memory and learning in rats. J. Nutr. Biochem. 2016, 31, 20–27. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, S.; Kling, D.E.; Leone, S.; Lawlor, N.T.; Huang, Y.; Feinberg, S.B.; Hill, D.R.; Newburg, D.S. The human milk oligosaccharide 2’-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut 2016, 65, 33–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo-Courtade, L.; Han, S.; Lee, S.; Mian, F.M.; Buck, R.; Forsythe, P. Attenuation of food allergy symptoms following treatment with human milk oligosaccharides in a mouse model. Allergy 2015, 70, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Newburg, D.S.; Ruiz-Palacios, G.M.; Morrow, A.L. Human milk glycans protect infants against enteric pathogens. Annu. Rev. Nutr. 2005, 25, 37–58. [Google Scholar] [CrossRef]
- Morrow, A.L.; Ruiz-Palacios, G.M.; Jiang, X.; Newburg, D.S. Human-milk glycans that inhibit pathogen binding protect breast-feeding infants against infectious diarrhea. J. Nutr. 2005, 135, 1304–1307. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Nanthakumar, N.N.; Newburg, D.S. The Human Milk Oligosaccharide 2’-Fucosyllactose Quenches Campylobacter jejuni-Induced Inflammation in Human Epithelial Cells HEp-2 and HT-29 and in Mouse Intestinal Mucosa. J. Nutr. 2016, 146, 1980–1990. [Google Scholar] [CrossRef]
- Coppa, G.V.; Facinelli, B.; Magi, G.; Marini, E.; Zampini, L.; Mantovani, V.; Galeazzi, T.; Padella, L.; Marchesiello, R.L.; Santoro, L.; et al. Human milk glycosaminoglycans inhibit in vitro the adhesion of Escherichia coli and Salmonella fyris to human intestinal cells. Pediatr. Res. 2016, 79, 603–607. [Google Scholar] [CrossRef] [Green Version]
- Weichert, S.; Jennewein, S.; Huefner, E.; Weiss, C.; Borkowski, J.; Putze, J.; Schroten, H. Bioengineered 2’-fucosyllactose and 3-fucosyllactose inhibit the adhesion of Pseudomonas aeruginosa and enteric pathogens to human intestinal and respiratory cell lines. Nutr. Res. 2013, 33, 831–838. [Google Scholar] [CrossRef]
- Ruiz-Palacios, G.M.; Cervantes, L.E.; Ramos, P.; Chavez-Munguia, B.; Newburg, D.S. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J. Biol. Chem. 2003, 278, 14112–14120. [Google Scholar] [CrossRef] [Green Version]
- Zopf, D.; Roth, S. Oligosaccharide anti-infective agents. Lancet 1996, 347, 1017–1021. [Google Scholar] [CrossRef]
- Newburg, D.S. Oligosaccharides in human milk and bacterial colonization. J Pediatr. Gastroenterol. Nutr. 2000, 30, S8–S17. [Google Scholar] [CrossRef] [PubMed]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; Van’t Land, B.; Stahl, B.; Garssen, J.; Jose Rodriguez-Lagunas, M.; Franch, A.; Castell, M.; Perez-Cano, F.J. Supplementation With 2’-FL and scGOS/lcFOS Ameliorates Rotavirus-Induced Diarrhea in Suckling Rats. Front. Cell. Infect. Microbiol. 2018, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Facinelli, B.; Marini, E.; Magi, G.; Zampini, L.; Santoro, L.; Catassi, C.; Monachesi, C.; Gabrielli, O.; Coppa, G.V. Breast milk oligosaccharides: Effects of 2’-fucosyllactose and 6’-sialyllactose on the adhesion of Escherichia coli and Salmonella fyris to Caco-2 cells. J. Matern. Fetal Neonatal Med. 2018, 32, 2950–2952. [Google Scholar] [CrossRef]
- Coppa, G.V.; Zampini, L.; Galeazzi, T.; Facinelli, B.; Ferrante, L.; Capretti, R.; Orazio, G. Human milk oligosaccharides inhibit the adhesion to Caco-2 cells of diarrheal pathogens: Escherichia coli, Vibrio cholerae, and Salmonella fyris. Pediatr. Res. 2006, 59, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Morrow, A.L.; Ruiz-Palacios, G.M.; Altaye, M.; Jiang, X.; Guerrero, M.L.; Meinzen-Derr, J.K.; Farkas, T.; Chaturvedi, P.; Pickering, L.K.; Newburg, D.S. Human milk oligosaccharide blood group epitopes and innate immune protection against campylobacter and calicivirus diarrhea in breastfed infants. Adv. Exp. Med. Biol. 2004, 554, 443–446. [Google Scholar]
- Peterson, R.; Cheah, W.Y.; Grinyer, J.; Packer, N. Glycoconjugates in human milk: Protecting infants from disease. Glycobiology 2013, 23, 1425–1438. [Google Scholar] [CrossRef] [Green Version]
- Bosscher, D.; Van Loo, J.; Franck, A. Inulin and oligofructose as prebiotics in the prevention of intestinal infections and diseases. Nutr. Res. Rev. 2006, 19, 216–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bovee-Oudenhoven, I.M.; Ten, B.S.; Lettink-Wissink, M.L.; van der Meer, R. Dietary fructo-oligosaccharides and lactulose inhibit intestinal colonisation but stimulate translocation of salmonella in rats. Gut 2003, 52, 1572–1578. [Google Scholar] [CrossRef] [Green Version]
- Ranucci, G.; Buccigrossi, V.; Borgia, E.; Piacentini, D.; Visentin, F.; Cantarutti, L.; Baiardi, P.; Felisi, M.; Spagnuolo, M.I.; Zanconato, S.; et al. Galacto-Oligosaccharide/Polidextrose Enriched Formula Protects against Respiratory Infections in Infants at High Risk of Atopy: A Randomized Clinical Trial. Nutrients 2018, 10, 286. [Google Scholar] [CrossRef] [Green Version]
- Zabel, B.; Yde, C.C.; Roos, P.; Marcussen, J.; Jensen, H.M.; Salli, K.; Hirvonen, J.; Ouwehand, A.C.; Morovic, W. Novel Genes and Metabolite Trends in Bifidobacterium longum subsp. infantis Bi-26 Metabolism of Human Milk Oligosaccharide 2’-fucosyllactose. Sci. Rep. 2019, 9, 7983. [Google Scholar] [CrossRef] [PubMed]
- Elison, E.; Vigsnaes, L.K.; Krogsgaard, L.R.; Rasmussen, J.; Sorensen, N.; McConnell, B.; Hennet, T.; Sommer, M.O.A.; Bytzer, P. Oral supplementation of healthy adults with 2-O-fucosyllactose and lacto-N-neotetraose is well tolerated and shifts the intestinal microbiota. Br. J. Nutr. 2016, 116, 1356–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Chen, C.; Kling, D.E.; Liu, B.; McCoy, J.M.; Merighi, M.; Heidtman, M.; Newburg, D.S. The principal fucosylated oligosaccharides of human milk exhibit prebiotic properties on cultured infant microbiota. Glycobiology 2013, 23, 169–177. [Google Scholar] [CrossRef]
- Gruenheid, S.; DeVinney, R.; Bladt, F.; Goosney, D.; Gelkop, S.; Gish, G.D.; Pawson, T.; Finlay, B.B. Enteropathogenic E-coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat. Cell Biol. 2001, 3, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Orskov, F.; Orskov, I. Escherichia coli serotyping and disease in man and animals. Can. J. Microbiol. 1992, 38, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Wadolkowski, E.A.; Burris, J.A.; O’Brien, A.D. Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 1990, 58, 2438–2445. [Google Scholar] [CrossRef] [Green Version]
- Asshauer, K.P.; Wemheuer, B.; Daniel, R.; Meinicke, P. Tax4Fun: Predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics 2015, 31, 2882–2884. [Google Scholar] [CrossRef]
- Rigothier, M.C.; Coconnier, M.H.; Servin, A.L.; Gayral, P. A new in vitro model of Entamoeba histolytica adhesion, using the human colon carcinoma cell line Caco-2: Scanning electron microscopic study. Infect. Immun. 1991, 59, 4142–4146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melton-Celsa, A.R. Shiga Toxin (Stx) Classification, Structure, and Function. Microbiol. Spectr. 2014, 2, 24–2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruzzese, E.; Volpicelli, M.; Squeglia, V.; Bruzzese, D.; Salvini, F.; Bisceglia, M.; Lionetti, P.; Cinquetti, M.; Iacono, G.; Amarri, S.; et al. A formula containing galacto- and fructo-oligosaccharides prevents intestinal and extra-intestinal infections: An observational study. Clin. Nutr. 2009, 28, 156–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reverri, E.; Devitt, A.; Kajzer, J.; Baggs, G.; Borschel, M. Review of the Clinical Experiences of Feeding Infants Formula Containing the Human Milk Oligosaccharide 2’-Fucosyllactose. Nutrients 2018, 10, 1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azagra-Boronat, I.; Massot-Cladera, M.; Mayneris-Perxachs, J.; Knipping, K.; Van’t Land, B.; Tims, S.; Stahl, B.; Garssen, J.; Franch, A.; Castell, M.; et al. Immunomodulatory and Prebiotic Effects of 2’-Fucosyllactose in Suckling Rats. Front. Immunol. 2019, 10, 1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azagra-Boronat, I.; Massot-Cladera, M.; Castell, M.; Rodriguez-Lagunas, M.J.; Van’t Land, B.; Knipping, K.; Garssen, J.; Perez-Cano, F.J. The Human Milk Oligosaccharide 2’-Fucosyllactose Ameliorates Rotavirus-Induced Diarrhoea in a Suckling Rat Model. Ann. Nutr. Metab. 2018, 732, 72. [Google Scholar]
- Ofek, I.; Sharon, N. Adhesins as lectins: Specificity and role in infection. Curr. Top. Microbiol. Immunol. 1990, 151, 91–113. [Google Scholar]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the Genetic Basis of Fibrolytic Specialization by Lachnospiraceae and Ruminococcaceae in Diverse Gut Communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Meehan, C.J.; Beiko, R.G. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol. Evol. 2014, 6, 703–713. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Chen, L.; Zhou, R.; Wang, X.; Song, L.; Huang, S.; Wang, G.; Xia, B. Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. J. Clin. Microbiol. 2014, 52, 398–406. [Google Scholar] [CrossRef] [Green Version]
- Vita, N.; Borne, R.; Perret, S.; de Philip, P.; Fierobe, H.P. Turning a potent family-9 free cellulase into an operational cellulosomal component and vice versa. FEBS J. 2019, 286, 3359–3373. [Google Scholar] [CrossRef] [PubMed]
- Cerisy, T.; Souterre, T.; Torres-Romero, I.; Boutard, M.; Dubois, I.; Patrouix, J.; Labadie, K.; Berrabah, W.; Salanoubat, M.; Doring, V.; et al. Evolution of a Biomass-Fermenting Bacterium to Resist Lignin Phenolics. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuntz, S.; Kunz, C.; Borsch, C.; Vazquez, E.; Buck, R.; Reutzel, M.; Eckert, G.P.; Rudloff, S. Metabolic Fate and Distribution of 2’-Fucosyllactose: Direct Influence on Gut Microbial Activity but not on Brain. Mol. Nutr. Food Res. 2019, 63, 1900035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salli, K.; Anglenius, H.; Hirvonen, J.; Hibberd, A.A.; Ahonen, I.; Saarinen, M.T.; Tiihonen, K.; Maukonen, J.; Ouwehand, A.C. The effect of 2’-fucosyllactose on simulated infant gut microbiome and metabolites; a pilot study in comparison to GOS and lactose. Sci Rep. 2019, 9, 13232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunesova, V.; Lacroix, C.; Schwab, C. Fucosyllactose and L-fucose utilization of infant Bifidobacterium longum and Bifidobacterium kashiwanohense. BMC Microbiol. 2016, 16, 248. [Google Scholar] [CrossRef] [Green Version]
- Hills, R.J.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [Green Version]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef]
- Cornick, S.; Tawiah, A.; Chadee, K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers 2015, 3, e982426. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.J.; Khan, W.I. Goblet cells and mucins: Role in innate defense in enteric infections. Pathogens 2013, 2, 55–70. [Google Scholar] [CrossRef] [Green Version]
- McCole, D.F. IBD candidate genes and intestinal barrier regulation. Inflamm. Bowel Dis. 2014, 20, 1829–1849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, C.S.; Ban, M.; Choi, E.J.; Moon, H.G.; Jeon, J.S.; Kim, D.K.; Park, S.K.; Jeon, S.G.; Roh, T.Y.; Myung, S.J.; et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS ONE 2013, 8, e76520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seregin, S.S.; Golovchenko, N.; Schaf, B.; Chen, J.; Pudlo, N.A.; Mitchell, J.; Baxter, N.T.; Zhao, L.; Schloss, P.D.; Martens, E.C.; et al. NLRP6 Protects Il10(-/-) Mice from Colitis by Limiting Colonization of Akkermansia muciniphila. Cell Rep. 2017, 19, 2174. [Google Scholar]
- Zhong, Y.; Marungruang, N.; Fak, F.; Nyman, M. Effects of two whole-grain barley varieties on caecal SCFA, gut microbiota and plasma inflammatory markers in rats consuming low- and high-fat diets. Br. J. Nutr. 2015, 113, 1558–1570. [Google Scholar] [CrossRef] [Green Version]
- Van den Abbeele, P.; Gerard, P.; Rabot, S.; Bruneau, A.; El Aidy, S.; Derrien, M.; Kleerebezem, M.; Zoetendal, E.G.; Smidt, H.; Verstraete, W.; et al. Arabinoxylans and inulin differentially modulate the mucosal and luminal gut microbiota and mucin-degradation in humanized rats. Environ. Microbiol. 2011, 13, 2667–2680. [Google Scholar] [CrossRef]
- Pelpolage, S.W.; Goto, Y.; Nagata, R.; Fukuma, N.; Furuta, T.; Mizu, M.; Han, K.H.; Fukushima, M. Colonic fermentation of water soluble fiber fraction extracted from sugarcane (Sacchurum officinarum L.) bagasse in murine models. Food Chem. 2019, 292, 336–345. [Google Scholar] [CrossRef]
- Ottman, N. Host Immunostimulation and Substrate Utilization of the Gut Symbiont Akkermansia Muciniphila; Wageningen University: Wageningen, The Netherlands, 2015. [Google Scholar]







| Group | IL-6 (pg/mL) | IL-1β (pg/mL) | TNF-α (pg/mL) | |
|---|---|---|---|---|
| Serum | CK | 76.61 ± 6.02 a | 13.86 ± 1.45 a | 35.78 ± 6.19 a |
| MC | 165.87 ± 5.63 b | 37.42 ± 2.42 b | 78.24 ± 5.75 b | |
| FL | 100.85 ± 8.44 c | 17.59 ± 1.21 c | 48.99 ± 5.40 c | |
| Ileum | CK | 170.72 ± 13.00 a | 16.82 ± 0.97 a | 40.34 ± 3.19 a |
| MC | 210.68 ± 8.97 b | 42.03 ± 2.49 b | 77.89 ± 6.49 b | |
| FL | 191.19 ± 9.66 c | 28.67 ± 1.72 c | 49.26 ± 4.06 c | |
| Colon | CK | 74.95 ± 3.77 a | 14.64 ± 0.74 a | 36.77 ± 1.85 a |
| MC | 160.40 ± 8.06 b | 34.30 ± 1.72 b | 60.14 ± 3.03 b | |
| FL | 147.38 ± 7.40 a | 25.62 ± 1.29 c | 45.61 ± 2.29 c |
| Group | Acetic Acid (μmol/g) | Propionic Acid (μmol/g) | Butyric Acid (μmol/g) | Valeric Acid (μmol/g) |
|---|---|---|---|---|
| CK | 43.45 ± 5.92 a | 12.98 ± 2.37 a | 14.32 ± 1.90 a | 5.04 ± 0.73 a |
| MC | 30.06 ± 6.97 b | 6.74 ± 1.68 b | 7.51 ± 1.58 b | 3.92 ± 0.41 b |
| FL | 45.98 ± 4.97 a | 13.93 ± 1.90 a | 14.26 ± 1.71 a | 4.77 ± 0.30 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zou, Y.; Wang, J.; Ma, H.; Zhang, B.; Wang, S. The Protective Effects of 2’-Fucosyllactose Against E. Coli O157 Infection Are Mediated by the Regulation of Gut Microbiota and the Inhibition of Pathogen Adhesion. Nutrients 2020, 12, 1284. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12051284
Wang Y, Zou Y, Wang J, Ma H, Zhang B, Wang S. The Protective Effects of 2’-Fucosyllactose Against E. Coli O157 Infection Are Mediated by the Regulation of Gut Microbiota and the Inhibition of Pathogen Adhesion. Nutrients. 2020; 12(5):1284. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12051284
Chicago/Turabian StyleWang, Yuanyifei, Yan Zou, Jin Wang, Hui Ma, Bowei Zhang, and Shuo Wang. 2020. "The Protective Effects of 2’-Fucosyllactose Against E. Coli O157 Infection Are Mediated by the Regulation of Gut Microbiota and the Inhibition of Pathogen Adhesion" Nutrients 12, no. 5: 1284. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12051284