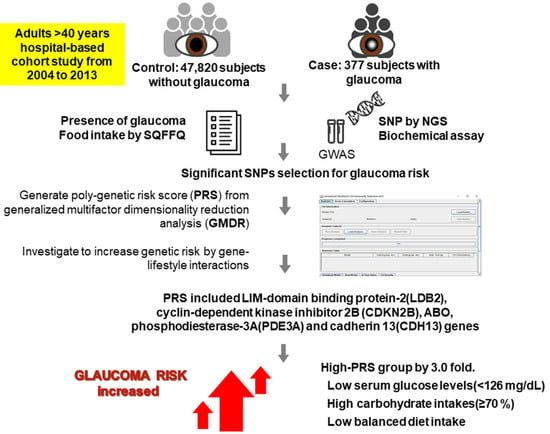
Polygenetic-Risk Scores for A Glaucoma Risk Interact with Blood Pressure, Glucose Control, and Carbohydrate Intake
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants Recruitment
2.2. Criteria of Glaucoma
2.3. Anthropometric and Biochemical Measurements
2.4. Assessment of Food and Nutrient Intakes Using a Semiquantitative Food Frequency Questionnaire (SQFFQ)
2.5. Genotyping and Quality Control
2.6. Identification of the Best Model for Gene-Gene Interactions by Generalized Multifactor Dimensionality Reduction (GMDR) Method among the Genetic Variants Selected by Logistic Regression
2.7. Statistical Analyses
3. Results
3.1. General Characteristics of Participants with Glaucoma
3.2. Association between Glaucoma Risk and Metabolic Syndrome
3.3. Selection of Genetic Variants Associated with Glaucoma Risk Using GMDR
3.4. A Positive Association between PRSs and Glaucoma Risk
3.5. Interaction between PRSs and Lifestyle in Glaucoma Risk
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Kametani, M.; Chen, D.F. Adaptive Immunity: New Aspects of Pathogenesis Underlying Neurodegeneration in Glaucoma and Optic Neuropathy. Front. Immunol. 2020, 11, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzybowski, A.; Och, M.; Kanclerz, P.; Leffler, C.; De Moraes, C.G. Primary Open Angle Glaucoma and Vascular Risk Factors: A Review of Population Based Studies from 1990 to 2019. J. Clin. Med. 2020, 9, 761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, Y.H.; Han, K.; Park, S.H.; Park, K.-M.; Yim, H.W.; Lee, W.-C.; Park, Y.G.; Park, Y.-M. Insulin Resistance Is Associated with Intraocular Pressure Elevation in a Non-Obese Korean Population. PLoS ONE 2015, 10, e112929. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Yasuda, M.; Ninomiya, T.; Hata, J.; Hashimoto, S.; Yoshitomi, T.; Kiyohara, Y.; Ishibashi, T. Insulin Resistance Is a Risk Factor for Increased Intraocular Pressure: The Hisayama Study. Investig. Opthalmology Vis. Sci. 2015, 56, 7983–7987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, B.J.; Aiello, L.P.; Pasquale, L.R. Presence and Risk Factors for Glaucoma in Patients with Diabetes. Curr. Diabetes Rep. 2016, 16, 124. [Google Scholar] [CrossRef]
- Zhao, D.; Cho, J.; Kim, M.H.; Friedman, D.S.; Guallar, E. Diabetes, Fasting Glucose, and the Risk of Glaucoma. Ophthalmol. 2015, 122, 72–78. [Google Scholar] [CrossRef]
- Dada, T. Is Glaucoma a Neurodegeneration caused by Central Insulin Resistance: Diabetes Type 4? J. Curr. Glaucoma Pr. Dvd. 2017, 11, 77–79. [Google Scholar] [CrossRef]
- Liu, H.; Qi, S.; He, W.; Chang, C.; Chen, Y.; Yu, J. Association of single-nucleotide polymorphisms in TLR4 gene and gene-environment interaction with primary open angle glaucoma in a Chinese northern population. J. Gene Med. 2019, 22, e3139. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Xing, Y.-Q.; Chen, Z.; Ma, X.-C.; Lu, Q. Association between interleukin-10 genetic polymorphisms and risk of primary open angle glaucoma in a Chinese Han population: A case-control study. Int. J. Ophthalmol. 2019, 12, 1605–1611. [Google Scholar] [CrossRef]
- Karmiris, E.; Kourtis, N.; Pantou, M.P.; Degiannis, D.; Georgalas, I.; Papaconstantinou, D. The Association between TGF-beta1 G915C (Arg25Pro) Polymorphism and the Development of Primary Open Angle Glaucoma: A Case-Control Study. Med. Hypothesis Discov. Innov. Ophthalmol. 2018, 7, 25–31. [Google Scholar] [PubMed]
- Ramdas, W.D.; Wolfs, R.C.W.; Jong, J.C.K.-D.; Hofman, A.; De Jong, P.T.V.M.; Vingerling, J.R.; Jansonius, N.M. Nutrient intake and risk of open-angle glaucoma: The Rotterdam Study. Eur. J. Epidemiol. 2012, 27, 385–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramdas, W.D.; Schouten, J.; Webers, C.A.B. The Effect of Vitamins on Glaucoma: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.H.; Pasquale, L.R.; Willett, W.C.; Rosner, B.; Egan, K.M.; Faberowski, N.; Hankinson, S. Dietary fat consumption and primary open-angle glaucoma. Am. J. Clin. Nutr. 2004, 79, 755–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, C.I.; Singh, K.; Lin, S. Relationship of lifestyle, exercise, and nutrition with glaucoma. Curr. Opin. Ophthalmol. 2019, 30, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ahn, J.; Lee, B.-K. Self-rated Subjective Health Status Is Strongly Associated with Sociodemographic Factors, Lifestyle, Nutrient Intakes, and Biochemical Indices, but Not Smoking Status: KNHANES 2007-2012. J. Korean Med. Sci. 2015, 30, 1279–1287. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Han, B.-G. The KoGES group Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017, 46, 1350. [Google Scholar] [CrossRef]
- Ahn, Y.; Kwon, E.; E Shim, J.; Park, M.K.; Joo, Y.; Kimm, K.; Park, C.; Kim, D.H. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef]
- Liu, M.; Jin, H.S.; Park, S. Protein and fat intake interacts with the haplotype of PTPN11_rs11066325, RPH3A_rs886477, and OAS3_rs2072134 to modulate serum HDL concentrations in middle-aged people. Clin. Nutr. 2020, 39, 942–949. [Google Scholar] [CrossRef]
- Rabbee, N.; Speed, T.P. A genotype calling algorithm for affymetrix SNP arrays. Bioinform. 2005, 22, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Jyothi, K.U.; Reddy, B.M. Gene-gene and gene-environment interactions in the etiology of type 2 diabetes mellitus in the population of Hyderabad, India. Meta Gene 2015, 5, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.-W.; Kim, S.H.; Zhang, X.; Park, S. Interactions among the variants of insulin-related genes and nutrients increase the risk of type 2 diabetes. Nutr. Res. 2018, 51, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Cho, J.; Kim, M.H.; Friedman, D.; Guallar, E. Diabetes, Glucose Metabolism, and Glaucoma: The 2005–2008 National Health and Nutrition Examination Survey. PLoS ONE 2014, 9, e112460. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Graham, S.L.; Pilowsky, P.M. Carbohydrate ingestion induces differential autonomic dysregulation in normal-tension glaucoma and primary open angle glaucoma. PLoS ONE 2018, 13, e0198432. [Google Scholar] [CrossRef]
- Balog, Z.; Sikić, J.; Vojniković, B.; Balog, S. Senile cataract and the absorption activity of cytochrome C oxidase. Coll. Antropol. 2001, 25. [Google Scholar]
- Liu, Y.C.; Wilkins, M.; Kim, T.; Malyugin, B.; Mehta, J.S. Cataracts. Lancet 2017, 390, 600–612. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, H.N.; Hwang, J.-Y.; Chang, N.; Kim, W.Y.; Chung, H.W.; Yang, Y.J. Development and evaluation of a food frequency questionnaire for Vietnamese female immigrants in Korea: The Korean Genome and Epidemiology Study (KoGES). Nutr. Res. Pr. 2011, 5, 260–265. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J. Dairy consumption is associated with a lower incidence of the metabolic syndrome in middle-aged and older Korean adults: The Korean Genome and Epidemiology Study (KoGES). Br. J. Nutr. 2017, 117, 148–160. [Google Scholar] [CrossRef] [Green Version]



| Parameters Related to Glaucoma | Non-Glaucoma (n = 47,820) | Glaucoma (n = 377) | Adjusted OR for Glaucoma Risk (OR, 95% CI) |
|---|---|---|---|
| Age (years) 1 | 53.7 ± 5.5 | 58.2 ± 5.4 *** | 3.325 (2.623–4.213) *** |
| Gender (number, male %) | 16,193 (33.9) | 171 (45.4) *** | 1.797 (1.430–2.257) *** |
| Education (number, %) | |||
| <High school | 6417 (18.5) | 66 (25.7) ** | 1 |
| High school | 7689 (22.1) | 60 (23.4) | 1.060 (0.724–1.552) |
| College more | 20,619 (59.4) | 131 (51.0) | 1.169 (0.821–1.665) |
| Income (number, %) | |||
| <$1000/m | 4508 (9.94) | 66 (18.5) *** | 1 |
| $1000–$2000 | 9722 (21.4) | 78 (21.9) | 0.699 (0.496–0.986) |
| $2000–$4000 | 20,047 (44.2) | 133 (37.4) | 0.756 (0.542–1.053) |
| >$4000 | 11,084 (24.4) | 79 (22.2) | 0.895 (0.607–1.320) |
| Exercise (number, %) | |||
| No | 21,531 (45.2) | 145 (38.7) * | 1 |
| Yes | 26,144 (54.8) | 230 (61.3) | 1.216 (0.971–1.523) |
| Alcohol intake (number, %) | |||
| No | 27,131 (56.7) | 238 (63.1) * | 1 |
| Mild drink (0–20 g) | 1048 (2.19) | 5 (1.33) | 0.656 (0.269–1.600) |
| Moderate drink (≥20 g) | 19,641 (41.1) | 134 (35.5) | 0.789 (0.612–1.016) |
| Coffee intake (number, %) | |||
| Low (<3 cups/week) | 18,037 (37.7) | 156 (41.4) | 1 |
| Medium (3–16 cups/week) | 29,329 (61.3) | 218 (57.8) | 1.030 (0.767–1.383) |
| High (≥16 cups/week) | 454 (0.95) | 3 (0.80) | 0.903 (0.704–1.157) |
| Energy intake 2 (kcal) | 1743 ± 531 | 1719 ± 516 | 0.848 (0.674–1.067) |
| CHO percent intake 3 | 71.7 ± 20.8 | 71.6 ± 20.0 | 1.037 (0.816–1.317) |
| Fat percent intake 4 | 13.9 ± 8.7 | 14.1 ± 8.0 | 1.137 (0.901–1.435) |
| Protein percent intake 5 | 13.4 ± 5.8 | 13.3 ± 5.6 | 0.919 (0.699–1.209) |
| Components for Metabolic Syndrome | Non-Glaucoma (n = 47,820) | Glaucoma (n = 377) | Adjusted OR for Glaucoma Risk (OR, 95% CI) |
|---|---|---|---|
| Metabolic syndrome 1 (number, %) | 6673 (14.0) | 81 (21.5) *** | 1.361 (1.032–1.793) # |
| BMI 2 (kg/m2) | 23.9 ± 2.8 | 23.8 ± 2.9 | 0.927 (0.736–1.167) |
| Waist circumferences 3 (cm) | 80.6 ± 8.7 | 80.8 ± 8.4 | 0.971 (0.751–1.255) |
| Serum glucose 4 (mg/dL) | 95.0 ± 20.2 | 100.1 ± 26.5 *** | 1.539 (1.182–2.003) ## |
| Blood HbA1c 5 (%) | 5.7 ± 0.7 | 5.9 ± 0.9 *** | 1.663 (1.170–2.364) ## |
| Serum total cholesterol 6 (mg/dL) | 197 ± 36 | 195 ± 38 | 1.108 (0.867–1.417) |
| Serum HDL 7 (mg/dL) | 54.4 ± 13.3 | 53.7 ± 12.8 | 1.181 (0.929–1.501) |
| Serum TG 8 (mg/dL) | 125 ± 86 | 119 ± 73 | 1.134 (0.901–1.428) |
| Serum BP 9 (number, %) | 11,627 (24.3) | 138 (36.6) *** | 1.225 (0.968–1.551) |
| Serum CRP-1 10 (mg/dL) | 0.14 ± 0.38 | 0.18 ± 0.45 | 2.066 (1.221–3.496) ## |
| CHR 1 | SNP 2 | Location | Mi 3 | OR 4 | p-Value for OR 5 | Genes | Feature | MAF 6 | HWE 7 |
|---|---|---|---|---|---|---|---|---|---|
| 4 | rs3763969 | 16648246 | T | 0.63 (0.48–0.83) | 9.7.E–04 | LDB2 | intron | 0.120 | 0.082 |
| 7 | rs1852542 | 42096521 | T | 1.67 (1.24–2.25) | 6.6.E–04 | GLI3 | intron | 0.041 | 0.559 |
| 8 | rs1020236 | 135543194 | C | 1.48 (1.19–1.83) | 4.4.E–04 | ZFAT | intron | 0.093 | 0.732 |
| 9 | rs523096 | 22019129 | G | 0.73 (0.57–0.93) | 1.0.E–02 | CDKN2B | intron | 0.134 | 0.972 |
| 9 | rs2073823 | 136132516 | A | 1.33 (1.13–1.56) | 7.6.E–04 | ABO | intron | 0.215 | 0.941 |
| 12 | rs12314390 | 20597977 | T | 1.70 (1.29–2.25) | 1.8.E–04 | PDE3A | intron | 0.048 | 0.558 |
| 13 | rs7335337 | 38221067 | G | 1.78 (1.34–2.37) | 7.8.E–05 | TRPC4 | intron | 0.041 | 0.230 |
| 15 | rs1319859 | 99230263 | G | 1.32 (1.13–1.53) | 3.5.E–04 | IGF1R | intron | 0.300 | 0.226 |
| 16 | rs12449180 | 83547527 | G | 1.42 (1.18–1.69) | 1.3.E–04 | CDH13 | intron | 0.162 | 0.162 |
| 18 | rs3902981 | 12658191 | G | 0.73 (0.62–0.87) | 3.6.E–04 | SPIRE1 | near-gene-5 | 0.300 | 0.492 |
| Glaucoma-Related Diseases | Adjustment 1 | Adjustment 2 | |||
|---|---|---|---|---|---|
| Low-PRS (n = 9245) | Medium-PRS (n = 31,227) | High-PRS (n = 6015) | Medium-PRS (n = 31,227) | High-PRS (n = 6015) | |
| Glaucoma | 1 | 1.814 (1.280–2.573) | 2.937 (1.965–4.389) *** | 1.815 (1.213–2.715) | 3.021 (1.898–4.809) *** |
| Cataract | 1 | 0.871 (0.767–0.988) | 0.935 (0.782–1.117) | 0.898 (0.771–1.045) | 0.983 (0.793–1.218) |
| Metabolic syndrome | 1 | 0.992 (0.924–1.064) | 1.004 (0.910–1.108) | 0.984 (0.891–1.086) | 1.037 (0.903–1.192) |
| Type 2 diabetes | 1 | 0.879 (0.805–0.959) * | 0.951 (0.841–1.075) | 0.873 (0.799–0.954) * | 0.930 (0.821–1.053) |
| Blood pressure | 1 | 1.006 (0.949–1.067) | 0.997 (0.919–1.082) | 0.979 (0.908–1.054) | 0.993 (0.894–1.103) |
| Parameters for Glaucoma Risk | Low-PRS (n = 14,420) | Medium-PRS (n = 21,641) | High-PRS (n = 4201) | Gene-Nutrient Interaction p-Value |
|---|---|---|---|---|
| Less aged people More aged people 1 | 1 | 1.575 (0.737–3.364) 1.907 (1.185–3.068) | 2.577 (1.063–6.246) * 3.187 (1.844–5.506) *** | 0.0092 |
| Low BP High BP 2 | 1 | 1.695 (1.024–2.805) 1.999 (1.021–3.915) | 3.659 (2.092–6.399) *** 1.751 (0.723–4.242) | 0.0106 |
| Low serum glucose High serum glucose 3 | 1 | 1.791 (1.149–2.719) 2.020 (0.770–5.297) | 3.165 (1.907–5.251) *** 2.195 (0.656–7.337) | 0.0460 |
| Low energy intake High energy intake 4 | 1 | 2.456(1.404–4.293) 1.210(0.669–2.191) | 3.959 (2.113–7.417) *** 1.432 (0.526–3.894)* | 0.1548 |
| Low CHO intake High CHO intake 5 | 1 | 1.685 (0.855–3.321) 1.892 (1.146–3.122) | 1.748 (0.722–4.236) 3.741 (2.139–6.544) *** | 0.0083 |
| Low protein intake High protein intake 6 | 1 | 2.134(1.316–3.459) 1.171(0.554–2.472) | 3.370(1.909–5.950) *** 3.229(1.885–5.532) | 0.2047 |
| Low fat intake High fat intake 7 | 1 | 1.743(1.053–2.887) 1.923(0.984–3.760) | 3.814(2.589–5.617) *** 2.440(1.076–5.536) | 0.1850 |
| Low Na intake High Na intake 8 | 1 | 1.725 (1.081–2.752) 2.030 (0.914–4.509) | 2.751 (1.594–4.749) *** 3.780 (1.542–9.266) ** | 0.7924 |
| Low BD intake High BD intake 9 | 1 | 2.244 (1.410–3.896) 0.980 (0.495–1.940) | 3.872(2.184–6.863) *** 1.700 (0.730–3.956) | 0.0464 |
| Low NBR intake High NBR intake 9 | 1 | 1.534 (0.946–2.488) 2.524 (1.207–5.281) * | 3.325 (1.934–5.717) *** 2.263 (0.903–5.672) | 0.1151 |
| Low RD intake High RD intake 9 | 1 | 1.794 (1.085–2.965) 1.855 (0.945–3.641) | 3.477 (1.970–6.139) *** 2.262 (0.997–5.132) | 0.4685 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jee, D.; Huang, S.; Kang, S.; Park, S. Polygenetic-Risk Scores for A Glaucoma Risk Interact with Blood Pressure, Glucose Control, and Carbohydrate Intake. Nutrients 2020, 12, 3282. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12113282
Jee D, Huang S, Kang S, Park S. Polygenetic-Risk Scores for A Glaucoma Risk Interact with Blood Pressure, Glucose Control, and Carbohydrate Intake. Nutrients. 2020; 12(11):3282. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12113282
Chicago/Turabian StyleJee, Donghyun, ShaoKai Huang, Suna Kang, and Sunmin Park. 2020. "Polygenetic-Risk Scores for A Glaucoma Risk Interact with Blood Pressure, Glucose Control, and Carbohydrate Intake" Nutrients 12, no. 11: 3282. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12113282