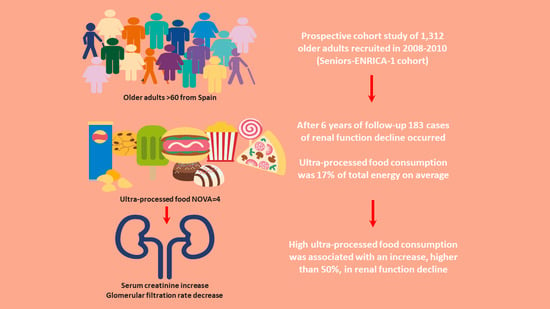
Ultra-Processed Food Consumption is Associated with Renal Function Decline in Older Adults: A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Variables
2.2.1. Diet and Covariables
Exposure Assessment and NOVA Classification
2.2.2. Renal Function Decline
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hommos, M.S.; Glassock, R.J.; Rule, A.D. Structural and functional changes in human kidneys with healthy aging. J. Am. Soc. Nephrol. 2017, 28, 2838–2844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar] [CrossRef] [Green Version]
- Bello, A.K.; Levin, A.; Tonelli, M.; Okpechi, I.G.; Feehally, J.; Harris, D.; Jindal, K.; Salako, B.L.; Rateb, A.; Osman, M.A.; et al. Assessment of global kidney health care status. JAMA J. Am. Med. Assoc. 2017, 317, 1864–1881. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Adetokunboh, O.; Ärnlöv, J.; Afshin, A.; et al. Global, regional, and national age-sex specifc mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef] [Green Version]
- Couser, W.G.; Remuzzi, G.; Mendis, S.; Tonelli, M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011, 80, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- Palmer, S.; Vecchio, M.; Craig, J.C.; Tonelli, M.; Johnson, D.W.; Nicolucci, A.; Pellegrini, F.; Saglimbene, V.; Logroscino, G.; Fishbane, S.; et al. Prevalence of depression in chronic kidney disease: Systematic review and meta-analysis of observational studies. Kidney Int. 2013, 84, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [Green Version]
- Rovin, B.H. Do kidneys grow old gracefully? Kidney Int. 2020, 97, 40–41. [Google Scholar] [CrossRef]
- Kelly, J.T.; Palmer, S.C.; Wai, S.N.; Ruospo, M.; Carrero, J.J.; Campbell, K.L.; Strippoli, G.F.M. Healthy dietary patterns and risk of mortality and ESRD in CKD: A meta-analysis of cohort studies. Clin. J. Am. Soc. Nephrol. 2017, 12, 272–279. [Google Scholar] [CrossRef]
- Luyckx, V.A.; Tuttle, K.R.; Garcia-Garcia, G.; Gharbi, M.B.; Heerspink, H.J.L.; Johnson, D.W.; Liu, Z.H.; Massy, Z.A.; Moe, O.; Nelson, R.G.; et al. Reducing major risk factors for chronic kidney disease. Kidney Int. Suppl. 2017, 7, 71–87. [Google Scholar] [CrossRef] [Green Version]
- Bach, K.E.; Kelly, J.T.; Campbell, K.L.; Palmer, S.C.; Khalesi, S.; Strippoli, G.F.M. Healthy dietary patterns and incidence of CKD: A meta-analysis of cohort studies. Clin. J. Am. Soc. Nephrol. 2019, 14, 1441–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juul, F.; Hemmingsson, E. Trends in consumption of ultra-processed foods and obesity in Sweden between 1960 and 2010. Public Health Nutr. 2015, 18, 3096–3107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandevijvere, S.; Moubarac, J.C.; Bentham, J.; Jaacks, L.M.; Monteiro, C.A.; Lee, A.C.; Butcher, M.G.; Swinburn, B. Global trends in ultraprocessed food and drink product sales and their association with adult body mass index trajectories. Obes. Rev. 2019, 20, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Machado, P.P.; Steele, E.M.; Levy, R.B.; Sui, Z.; Rangan, A.; Woods, J.; Gill, T.; Scrinis, G.; Monteiro, C.A. Ultra-processed foods and recommended intake levels of nutrients linked to non-communicable diseases in Australia: Evidence from a nationally representative cross-sectional study. BMJ Open 2019, 9, e029544. [Google Scholar] [CrossRef] [Green Version]
- Steele, E.M.; Baraldi, L.G.; Da Costa Louzada, M.L.; Moubarac, J.C.; Mozaffarian, D.; Monteiro, C.A. Ultra-processed foods and added sugars in the US diet: Evidence from a nationally representative cross-sectional study. BMJ Open 2016, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Rojo, R.; Sandoval-Insausti, H.; López-Garcia, E.; Graciani, A.; Ordovás, J.M.; Banegas, J.R.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Consumption of Ultra-Processed Foods and Mortality: A National Prospective Cohort in Spain. Mayo Clin. Proc. 2019, 94, 2178–2188. [Google Scholar] [CrossRef] [Green Version]
- Rico-Campà, A.; Martínez-González, M.A.; Alvarez-Alvarez, I.; De Deus Mendonça, R.; De La Fuente-Arrillaga, C.; Gómez-Donoso, C.; Bes-Rastrollo, M. Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ 2019, 365, l1949. [Google Scholar] [CrossRef] [Green Version]
- Ares, G.; Vidal, L.; Allegue, G.; Giménez, A.; Bandeira, E.; Moratorio, X.; Molina, V.; Curutchet, M.R. Consumers’ conceptualization of ultra-processed foods. Appetite 2016, 105, 611–617. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Yang, H.; Qiu, P.; Wang, H.; Wang, F.; Zhao, Q.; Fang, J.; Nie, J. Consumption of ultra-processed foods and health outcomes: A systematic review of epidemiological studies. Nutr. J. 2020, 19, 1–10. [Google Scholar] [CrossRef]
- Louzada, M.L.D.C.; Martins, A.P.B.; Canella, D.S.; Baraldi, L.G.; Levy, R.B.; Claro, R.M.; Moubarac, J.C.; Cannon, G.; Monteiro, C.A. Ultra-processed foods and the nutritional dietary profile in Brazil. Rev. Saude Publica 2015, 49, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Moubarac, J.C.; Martins, A.P.B.; Claro, R.M.; Levy, R.B.; Cannon, G.; Monteiro, C.A. Consumption of ultra-processed foods and likely impact on human health. Evidence from Canada. Public Health Nutr. 2013, 16, 2240–2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louzada, M.L.D.C.; Martins, A.P.B.; Canella, D.S.; Baraldi, L.G.; Levy, R.B.; Claro, R.M.; Moubarac, J.C.; Cannon, G.; Monteiro, C.A. Impact of ultra-processed foods on micronutrient content in the Brazilian diet. Rev. Saude Publica 2015, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cranston, J.M.; Crockett, A.J.; Moss, J.R.; Pegram, R.W.; Stocks, N.P. Ultra-Processed Food and Health Outcomes: A narrative review. Nutrients 2020, 12, 1955. [Google Scholar] [CrossRef] [PubMed]
- De Deus Mendonca, R.; Marcal, A.; Gea, A.; de la Fuente-Arrillaga, C.; Martinez-Gonzalez, M.A.; Souza Lopes, M.A.; Bess-Rastrollo, M. Ultraprocessed food consumption and risk of overweight and obesity: The University of Navarra Follow-Up (SUN) cohort study. Am. J. Clin. Nutr. 2017, 105, 1012. [Google Scholar] [CrossRef]
- Sandoval-Insausti, H.; Jiménez-Onsurbe, M.; Donat-Vargas, C.; Rey-García, J.; Banegas, J.R.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Ultra-Processed Food Consumption Is Associated with Abdominal Obesity: A Prospective Cohort Study in Older Adults. Nutrients 2020, 12, 2368. [Google Scholar] [CrossRef] [PubMed]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Allès, B.; Debras, C.; Druesne-Pecollo, N.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultraprocessed Food Consumption and Risk of Type 2 Diabetes among Participants of the NutriNet-Santé Prospective Cohort. JAMA Intern. Med. 2020, 180, 283–291. [Google Scholar] [CrossRef]
- De Deus Mendonça, R.; Souza Lopes, A.C.; Pimenta, A.M.; Gea, A.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Ultra-processed food consumption and the incidence of hypertension in a mediterranean cohort: The seguimiento universidad de navarra project. Am. J. Hypertens. 2017, 30, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Hu, E.; Rebholz, C.M. Ultra-processed food intake and mortality in the United States: Results from the Third National Health and Nutrition Examination Survey (NHANES III 1988–1994). Public Health Nutr. 2019, 22, 1777–1785. [Google Scholar] [CrossRef]
- Schnabel, L.; Kesse-Guyot, E.; Allès, B.; Touvier, M.; Srour, B.; Hercberg, S.; Buscail, C.; Julia, C. Association between Ultraprocessed Food Consumption and Risk of Mortality among Middle-aged Adults in France. JAMA Intern. Med. 2019, 179, 490–498. [Google Scholar] [CrossRef]
- Rodríguez-Artalejo, F.; Graciani, A.; Guallar-Castillón, P.; León-Muñoz, L.M.; Zuluaga, M.C.; López-García, E.; Gutiérrez-Fisac, J.L.; Taboada, J.M.; Aguilera, M.T.; Regidor, E.; et al. Justificación y métodos del estudio sobre nutrición y riesgo cardiovascular en España (ENRICA). Rev. Esp. Cardiol. 2011, 64, 876–882. [Google Scholar] [CrossRef]
- León-Muñoz, L.M.; García-Esquinas, E.; López-García, E.; Banegas, J.R.; Rodríguez-Artalejo, F. Major dietary patterns and risk of frailty in older adults: A prospective cohort study. BMC Med. 2015, 13, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guallar-Castillón, P.; Sagardui-Villamor, J.; Balboa-Castillo, T.; Sala-Vila, A.; Astolfi, M.J.A.; Pelous, M.D.S.; León-Muñoz, L.M.; Graciani, A.; Laclaustra, M.; Benito, C.; et al. Validity and reproducibility of a Spanish dietary history. PLoS ONE 2014, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, C.A.I. Relative Validity and Reproducibility of a Diet History Questionnaire in Spain I. Foods. EPIC Group of Spain. European Prospective Investigation into Cancer and Nutrition. Int. J. Epidemiol. 1997, 26, 91–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pols, M.A.; Peeters, P.H.; Ocke, M.C.; Slimani, N.; Bueno-de-Mesquita, H.B.; Collette, H.J. Estimation of reproducibility and relative validity of the questions included in the EPIC Physical Activity Questionnaire. Int. J. Epidemiol. 1997, 26 (Suppl. 1), S181–S189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, C.A.; Levy, R.B.; Claro, R.M.; de Castro, I.R.R.; Cannon, G. A new classification of foods based on the extent and purpose of their processing. Cad. Saude Publica 2010, 26, 2039–2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.C.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Bash, L.D.; Coresh, J.; Köttgen, A.; Parekh, R.S.; Fulop, T.; Wang, Y.; Astor, B.C. Defining incident chronic kidney disease in the research setting. Am. J. Epidemiol. 2009, 170, 414–424. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; Von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, 1628–1654. [Google Scholar] [CrossRef] [Green Version]
- Lachat, C.; Hawwash, D.; Ocké, M.C.; Berg, C.; Forsum, E.; Hörnell, A.; Larsson, C.; Sonestedt, E.; Wirfält, E.; Åkesson, A.; et al. Strengthening the Reporting of Observational Studies in Epidemiology—Nutritional Epidemiology (STROBE-nut): An Extension of the STROBE Statement. PLoS Med. 2016, 13, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Hörnell, A.; Berg, C.; Forsum, E.; Larsson, C.; Sonestedt, E.; Åkesson, A.; Lachat, C.; Hawwash, D.; Kolsteren, P.; Byrnes, G.; et al. Perspective: An Extension of the STROBE Statement for Observational Studies in Nutritional Epidemiology (STROBE-nut): Explanation and Elaboration. Adv. Nutr. An. Int. Rev. J. 2017, 8, 652–678. [Google Scholar] [CrossRef] [PubMed]
- Khatri, M.; Moon, Y.P.; Scarmeas, N.; Gu, Y.; Gardener, H.; Cheung, K.; Wright, C.B.; Sacco, R.L.; Nickolas, T.L.; Elkind, M.S.V. The association between a mediterranean-style diet and kidney function in the northern manhattan study cohort. Clin. J. Am. Soc. Nephrol. 2014, 9, 1868–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebholz, C.M.; Crews, D.C.; Grams, M.E.; Steffen, L.M.; Levey, A.S.; Miller, E.R.; Appel, L.J.; Coresh, J. DASH (Dietary Approaches to Stop Hypertension) Diet and Risk of Subsequent Kidney Disease. Am. J. Kidney Dis. 2016, 68, 853–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Caulfield, L.E.; Garcia-Larsen, V.; Steffen, L.M.; Grams, M.E.; Coresh, J.; Rebholz, C.M. Plant-based diets and incident CKD and kidney function. Clin. J. Am. Soc. Nephrol. 2019, 14, 682–691. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, F.S.; Dias, M.D.S.; Mintem, G.C.; De Oliveira, I.O.; Gigante, D.P. Food processing and cardiometabolic risk factors: A systematic review. Rev. Saude Publica 2020, 54, 70. [Google Scholar] [CrossRef]
- Mirmiran, P.; Yuzbashian, E.; Asghari, G.; Sarverzadeh, S.; Azizi, F. Dietary fibre intake in relation to the risk of incident chronic kidney disease. Br. J. Nutr. 2018, 119, 479–485. [Google Scholar] [CrossRef] [Green Version]
- Fioretto, P.; Bruseghin, M.; Berto, I.; Gallina, P.; Manzato, E.; Mussap, M. Renal protection in diabetes: Role of glycemic control. J. Am. Soc. Nephrol. 2006, 17, 86–89. [Google Scholar] [CrossRef] [Green Version]
- Aleixandre, A.; Miguel, M. Dietary fiber and blood pressure control. Food Funct. 2016, 7, 1864–1871. [Google Scholar] [CrossRef]
- Yuzbashian, E.; Asghari, G.; Mirmiran, P.; Amouzegar-Bahambari, P.; Azizi, F. Adherence to low-sodium Dietary Approaches to Stop Hypertension-style diet may decrease the risk of incident chronic kidney disease among high-risk patients: A secondary prevention in prospective cohort study. Nephrol. Dial. Transpl. 2018, 33, 1159–1168. [Google Scholar] [CrossRef]
- Yoon, C.Y.; Noh, J.; Lee, J.; Kee, Y.K.; Seo, C.; Lee, M.; Cha, M.U.; Kim, H.; Park, S.; Yun, H.R.; et al. High and low sodium intakes are associated with incident chronic kidney disease in patients with normal renal function and hypertension. Kidney Int. 2018, 93, 921–931. [Google Scholar] [CrossRef] [Green Version]
- Graudal, N. U-shaped dietary sodium–associated incidence of chronic kidney disease cautions against salt overrestriction in hypertension. Kidney Int. 2018, 93, 776–778. [Google Scholar] [CrossRef] [PubMed]
- McMahon, E.J.; Campbell, K.L.; Bauer, J.D.; Mudge, D.W. Altered dietary salt intake for people with chronic kidney disease Sodium restriction in heart failure: A rapid review. Cochrane Database Syst. Rev. 2015, 2. [Google Scholar] [CrossRef]
- Yuzbashian, E.; Asghari, G.; Mirmiran, P.; Zadeh-Vakili, A.; Azizi, F. Sugar-sweetened beverage consumption and risk of incident chronic kidney disease: Tehran lipid and glucose study. Nephrology 2016, 21, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Rebholz, C.M.; Young, B.A.; Katz, R.; Tucker, K.L.; Carithers, T.C.; Norwood, A.F.; Correa, A. Patterns of beverages consumed and risk of incident kidney disease. Clin. J. Am. Soc. Nephrol. 2019, 14, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio-Aliaga, I. Phosphate and kidney healthy aging. Kidney Blood Press. Res. 2020, 8057, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; De Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Gutekunst, L.; Mehrotra, R.; Kovesdy, C.P.; Bross, R.; Shinaberger, C.S.; Noori, N.; Hirschberg, R.; Benner, D.; Nissenson, A.R.; et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 519–530. [Google Scholar] [CrossRef]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gürtler, R.; Husøy, T.; Mennes, W.; et al. Re-evaluation of phosphoric acid–phosphates—di-, tri- and polyphosphates (E 338–341, E 343, E 450–452) as food additives and the safety of proposed extension of use. EFSA J. 2019, 17, e05674. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, O.M. Sodium- and Phosphorus-Based Food Additives: Persistent but Surmountable Hurdles in the Management of Nutrition in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2013, 20, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Uribarri, J. Phosphorus additives in food and their effect in dialysis patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1290–1292. [Google Scholar] [CrossRef] [Green Version]
- Dudley, J.; Blackburn, C. Extraskeletal Calcifications complicating oral neutral-phosphate therapy. Lancet 1970, 296, 628–630. [Google Scholar] [CrossRef]
- Adeney, K.L.; Siscovick, D.S.; Ix, J.H.; Seliger, S.L.; Shlipak, M.G.; Jenny, N.S.; Kestenbaum, B.R. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J. Am. Soc. Nephrol. 2009, 20, 381–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, S.; Trivedi, B.K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Association of disorders in mineral metabolism with progression of chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2006, 1, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, J.; Scanni, R.; Bestmann, L.; Hulter, H.N.; Krapf, R. A controlled increase in dietary phosphate elevates BP in healthy human subjects. J. Am. Soc. Nephrol. 2018, 29, 2089–2098. [Google Scholar] [CrossRef] [Green Version]
- Mancini, F.R.; Affret, A.; Dow, C.; Balkau, B.; Clavel-Chapelon, F.; Bonnet, F.; Boutron-Ruault, M.C.; Fagherazzi, G. High dietary phosphorus intake is associated with an increased risk of type 2 diabetes in the large prospective E3N cohort study. Clin. Nutr. 2018, 37, 1625–1630. [Google Scholar] [CrossRef]


| Ultra-Processed Food Consumption (% energy) | ||||
|---|---|---|---|---|
| T1 (Lowest) (n = 438) | T2 (n = 438) | T3 (Highest) (n = 436) | p Trend | |
| Total energy (kcal/day), mean ± SD | 1948 ± 549 | 2053 ± 565 | 2161 ± 569 | <0.001 |
| Ultra-processed food consumption (% energy), mean ± SD | 7.7 ± 3.5 | 17.5 ± 3.0 | 31.5 ± 7.7 | <0.001 |
| Ultra-processed food consumption (grams per day), mean ± SD | 128 ± 99 | 251 ± 141 | 379 ± 177 | <0.001 |
| Weight (kg), mean ± SD | 73.7 ± 13 | 74.8 ± 13 | 76.0 ± 13.0 | 0.044 |
| Ultra-processed food consumption (g/kg), mean ± SD | 1.8 ± 1.3 | 3.4 ± 1.9 | 5.1 ± 2.5 | <0.001 |
| Age, years, mean ± SD | 67.4 ± 5.5 | 67 ± 5.2 | 67 ± 5.8 | 0.823 |
| Educational level, % | 0.735 † | |||
| No formal education or primary | 23.7 | 23.9 | 23.9 | |
| Secondary | 25.6 | 25.8 | 29.1 | |
| University | 50.7 | 50.2 | 47.0 | |
| Smoking status, % | 0.356 † | |||
| Never smoker | 57.8 | 58.9 | 54.6 | |
| Former smoker | 32.2 | 28.3 | 32.1 | |
| Current Smoker | 10.1 | 12.8 | 13.3 | |
| Former-drinker status, % | 8.7 | 4.6 | 10.8 | 0.003 † |
| Physical activity, MET-hour/week, mean ± SD | 63 ± 34 | 60 ± 32 | 58 ± 34 | 0.035 |
| Time spent watching TV, hour/week, mean ± SD | 2.4 ± 1.5 | 2.5 ± 1.5 | 2.4 ± 1.6 | 0.500 |
| Fiber (grams/day), mean ± SD | 24.4 ± 8.0 | 25.1 ± 8.0 | 24 ± 7.6 | 0.477 |
| Number of chronic conditions, mean ± SD | 0.7 ± 0.7 | 0.7 ± 0.7 | 0.7 ± 0.8 | 0.400 |
| Number of medications per day, mean ± SD | 1.7 ± 1.7 | 1.8 ± 1.8 | 1.7 ± 1.9 | 0.389 |
| Hypertension, % | 63.7 | 64.3 | 56.4 | 0.118 † |
| Diabetes mellitus, % | 13 | 12.8 | 15.3 | 0.471 † |
| Hypercholesterolemia, % | 70.8 | 72.1 | 73.6 | 0.643 |
| BMI baseline, mean ± SD | 28.1 ± 4 | 28.5 ± 4.4 | 28.6 ± 4.2 | 0.124 |
| T1 (Lowest) OR (95% CI) | T2 OR (95% CI) | T3 (Highest) OR (95% CI) | p Trend | |
|---|---|---|---|---|
| Ultra-Processed Food Consumption (% Energy) | ||||
| n | 438 | 438 | 436 | |
| Cases | 47 | 67 | 69 | |
| Model 1 | Ref. | 1.63 (1.08–2.44) | 1.75 (1.16–2.64) | 0.008 |
| Model 2 | Ref. | 1.56 (1.04–2.35) | 1.69 (1.11–2.55) | 0.014 |
| Model 3 | Ref. | 1.56 (1.02–2.38) | 1.74 (1.14–2.66) | 0.026 |
| Ultra-Processed Food Consumption (g/kg/Day) | ||||
| n | 438 | 437 | 437 | |
| Cases | 55 | 61 | 67 | |
| Model 1 | Ref. | 1.26 (0.84–1.89) | 1.56 (1.03–2.35) | 0.034 |
| Model 2 | Ref. | 1.25 (0.84–1.88) | 1.57 (1.04–2.38) | 0.033 |
| Model 3 | Ref. | 1.28 (0.85–1.95) | 1.62 (1.06–2.49) | 0.043 |
| Ultra-Processed Food Consumption (% Energy) | ||||
|---|---|---|---|---|
| T1 (Lowest) | T2 | T3 (Highest) | p Trend | |
| With at least one chronic condition | ||||
| n/cases | 229/25 | 241/38 | 232/38 | |
| OR (95% CI) | 1 (Ref.) | 1.49 (0.85–2.62) | 1.5(0.84–2.68) | 0.174 |
| Without any chronic condition | ||||
| n/cases | 209/22 | 197/29 | 204/31 | |
| OR (95% CI) | 1 (Ref.) | 1.47 (0.78–2.76) | 1.61 (0.86–3.03) | 0.137 |
| With hypertension | ||||
| n/cases | 279/33 | 282/48 | 246/45 | |
| OR (95% CI) | 1 (Ref.) | 1.54 (0.94–2.53) | 1.65 (0.99–2.75) | 0.055 |
| Without hypertension | ||||
| n/cases | 159/14 | 156/19 | 190/24 | |
| OR (95% CI) | 1 (Ref.) | 1.49 (0.68–3.24) | 1.52 (0.70–3.27) | 0.305 |
| With diabetes | ||||
| n/cases | 57/8 | 56/14 | 67/22 | |
| OR (95% CI) | 1 (Ref.) | 1.86 (0.62–5.6) | 3.08 (1.08–8.75) | 0.034 |
| Without diabetes | ||||
| n/cases | 381/39 | 382/53 | 369/47 | |
| OR (95% CI) | 1 (Ref.) | 1.43 (0.91–2.25) | 1.36 (0.85–2.19) | 0.200 |
| With hypercholesterolemia | ||||
| n/cases | 310/34 | 316/47 | 321/53 | |
| OR (95% CI) | 1 (Ref.) | 1.50 (0.92–2.46) | 1.67 (1.03–2.73) | 0.042 |
| Without hypercholesterolemia | ||||
| n/cases | 128/13 | 122/20 | 115/16 | |
| OR (95% CI) | 1 (Ref.) | 1.63 (0.72–3.70) | 1.38 (0.59–3.27) | 0.474 |
| With obesity (BMI ≥ 30 kg/m2) | ||||
| n/cases | 125/17 | 141/27 | 128/18 | |
| OR (95% CI) | 1 (Ref.) | 1.55 (0.76–3.14) | 1.08 (0.50–2.32) | 0.833 |
| Without obesity (BMI < 30 kg/m2) | ||||
| n/cases | 313/30 | 297/40 | 308/51 | |
| OR (95% CI) | 1 (Ref.) | 1.49 (0.88–2.53) | 1.90 (1.13–3.19) | 0.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rey-García, J.; Donat-Vargas, C.; Sandoval-Insausti, H.; Bayan-Bravo, A.; Moreno-Franco, B.; Banegas, J.R.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Ultra-Processed Food Consumption is Associated with Renal Function Decline in Older Adults: A Prospective Cohort Study. Nutrients 2021, 13, 428. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020428
Rey-García J, Donat-Vargas C, Sandoval-Insausti H, Bayan-Bravo A, Moreno-Franco B, Banegas JR, Rodríguez-Artalejo F, Guallar-Castillón P. Ultra-Processed Food Consumption is Associated with Renal Function Decline in Older Adults: A Prospective Cohort Study. Nutrients. 2021; 13(2):428. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020428
Chicago/Turabian StyleRey-García, Jimena, Carolina Donat-Vargas, Helena Sandoval-Insausti, Ana Bayan-Bravo, Belén Moreno-Franco, José Ramón Banegas, Fernando Rodríguez-Artalejo, and Pilar Guallar-Castillón. 2021. "Ultra-Processed Food Consumption is Associated with Renal Function Decline in Older Adults: A Prospective Cohort Study" Nutrients 13, no. 2: 428. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020428