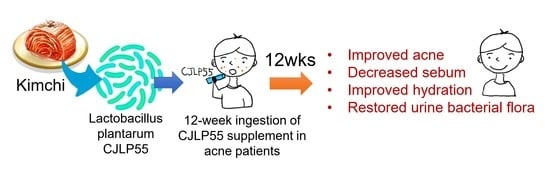
Effects of Lactobacillus plantarum CJLP55 on Clinical Improvement, Skin Condition and Urine Bacterial Extracellular Vesicles in Patients with Acne Vulgaris: A Randomized, Double-Blind, Placebo-Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Probiotics and Placebo Products
2.2. Study Subjects
2.3. Study Design
2.4. Clinical Assessment of Facial Acne
2.5. Measurement of Skin Condition-Related Factors
2.6. Analysis of Skin Surface Lipids
2.7. Metagenomic DNA Analysis of Bacterial EV Phylum in Urine
2.8. Statistics
3. Results
3.1. Study Subjects
3.2. Clinical Assessment of Facial Acne
3.3. Measurement of Skin Condition-Related Factors
3.4. Analysis of Skin Surface Lipids
3.5. Metagenomic DNA Analysis of Bacterial EV Phylum in Urine
3.6. Correlations between Percent Changes in TG Levels and in Proteobacteria and Firmicutes Proportions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurokawa, I.; Danby, F.W.; Ju, Q.; Wang, X.; Xiang, L.F.; Xia, L.; Chen, W.C.; Nagy, I.; Picardo, M.; Suh, D.H.; et al. New developments in our understanding of acne pathogenesis and treatment. Exp. Dermatol. 2009, 18, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, M.; Morohashi, M. Pathogenesis of acne. Med. Electron. Microsc. 2001, 34, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Bowe, W.P.; Logan, A.C. Clinical implications of lipid peroxidation in acne vulgaris: Old wine in new bottles. Lipids Health Dis. 2010, 9, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, M.; Bowe, W.P.; Heughebaert, C.; Shalita, A.R. The development of antimicrobial resistance due to the antibiotic treatment of acne vulgaris: A review. J. Drugs Dermatol. 2010, 9, 655–664. [Google Scholar]
- Lee, H.; Yoon, H.; Ji, Y.; Kim, H.; Park, H.; Lee, J.; Shin, H.; Holzapfel, W. Functional properties of Lactobacillus strains isolated from kimchi. Int. J. Food Microbiol. 2011, 145, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Kim, H.; Park, H.; Lee, J.; Lee, H.; Shin, H.; Kim, B.; Franz, C.M.A.P.; Holzapfel, W.H. Functionality and safety of lactic bacterial strains from Korean kimchi. Food Control. 2013, 31, 467–473. [Google Scholar] [CrossRef]
- Kober, M.M.; Bowe, W.P. The effect of probiotics on immune regulation, acne, and photoaging. Int. J. Women’s Dermatol. 2015, 1, 85–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouwehand, A.C.; Salminen, S.; Isolauri, E. Probiotics: An overview of beneficial effects. Antonie Van Leeuwenhoek 2002, 82, 279–289. [Google Scholar] [CrossRef]
- Dunne, C.; O’Mahony, L.; Murphy, L.; Thornton, G.; Morrissey, D.; O’Halloran, S.; Feeney, M.; Flynn, S.; Fitzgerald, G.; Daly, C.; et al. In vitro selection criteria for probiotic bacteria of human origin: Correlation with in vivo findings. Am. J. Clin. Nutr. 2001, 73, 386s–392s. [Google Scholar] [CrossRef] [Green Version]
- Won, T.J.; Kim, B.; Song, D.S.; Lim, Y.T.; Oh, E.S.; Lee, D.I.; Park, E.S.; Min, H.; Park, S.Y.; Hwang, K.W. Modulation of Th1/Th2 balance by Lactobacillus strains isolated from Kimchi via stimulation of macrophage cell line J774A.1 in vitro. J. Food Sci. 2011, 76, H55–H61. [Google Scholar] [CrossRef]
- Won, T.J.; Kim, B.; Lim, Y.T.; Song, D.S.; Park, S.Y.; Park, E.S.; Lee, D.I.; Hwang, K.W. Oral administration of Lactobacillus strains from Kimchi inhibits atopic dermatitis in NC/Nga mice. J. Appl. Microbiol. 2011, 110, 1195–1202. [Google Scholar] [CrossRef]
- Muizzuddin, N.; Maher, W.; Sullivan, M.; Schnittger, S.; Mammone, T. Physiological effect of a probiotic on skin. J. Cosmet. Sci. 2012, 63, 385–395. [Google Scholar]
- Han, Y.; Kim, B.; Ban, J.; Lee, J.; Kim, B.J.; Choi, B.S.; Hwang, S.; Ahn, K.; Kim, J. A randomized trial of Lactobacillus plantarum CJLP133 for the treatment of atopic dermatitis. Pediatr. Allergy Immunol. 2012, 23, 667–673. [Google Scholar] [CrossRef]
- Tsai, W.H.; Chou, C.H.; Chiang, Y.J.; Lin, C.G.; Lee, C.H. Regulatory effects of lactobacillus plantarum-GMNL6 on human skin health by improving skin microbiome. Int. J. Med. Sci. 2021, 18, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Pyun, B.Y. Extracellular vesicle: An unknown environmental factor for causing airway disease. Allergy Asthma Immunol. Res. 2016, 8, 179–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.H.; Choi, S.J.; Choi, H.I.; Choi, J.P.; Park, H.K.; Kim, E.K.; Kim, M.J.; Moon, B.S.; Min, T.K.; Rho, M.; et al. Lactobacillus plantarum-derived extracellular vesicles protect atopic dermatitis induced by Staphylococcus aureus-derived Extracellular Vesicles. Allergy Asthma Immunol. Res. 2018, 10, 516–532. [Google Scholar] [CrossRef] [PubMed]
- Courage + Khazaka Electronic GmbH, Cologne, G. The Sebumeter®SM815. Available online: https://www.courage-khazaka.de/en/scientific-products/all-products/16-wissenschaftliche-produkte/alle-produkte/151-sebumeter-e (accessed on 31 May 2016).
- Kim, J.; Ko, Y.; Park, Y.K.; Kim, N.I.; Ha, W.K.; Cho, Y. Dietary effect of lactoferrin-enriched fermented milk on skin surface lipid and clinical improvement of acne vulgaris. Nutrition 2010, 26, 902–909. [Google Scholar] [CrossRef]
- Clarys, P.; Barel, A. Quantitative evaluation of skin surface lipids. Clin. Dermatol. 1995, 13, 307–321. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Rho, M.; You, Y.A.; Kwon, E.J.; Kim, M.H.; Kym, S.; Jee, Y.K.; Kim, Y.K.; Kim, Y.J. 16S rRNA gene-based metagenomic analysis reveals differences in bacteria-derived extracellular vesicles in the urine of pregnant and non-pregnant women. Exp. Mol. Med. 2016, 48, e208. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Park, J.Y.; Lee, E.H.; Yang, J.; Jeong, B.R.; Kim, Y.K.; Seoh, J.Y.; Lee, S.H.; Han, P.L.; Kim, E.J. Rapid assessment of microbiota changes in individuals with autism spectrum disorder using bacteria-derived membrane vesicles in urine. Exp. Neurobiol. 2017, 26, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.C.; Mendelsohn, R.; Rerek, M.E.; Moore, D.J. Fourier transform infrared spectroscopy and differential scanning calorimetry studies of fatty acid homogeneous ceramide 2. Biochim. Biophys. Acta Biomembr. 2000, 1468, 293–303. [Google Scholar] [CrossRef] [Green Version]
- Cha, H.M.; Kim, S.K.; Kook, M.C.; Yi, T.H. Lactobacillus paraplantarum THG-G10 as a potential anti-acne agent with anti-bacterial and anti-inflammatory activities. Anaerobe 2020, 64, 102243. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.H.; Yoon, J.Y.; Hong, J.S.; Jung, J.Y.; Park, M.S.; Suh, D.H. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: A randomized, controlled trial. Acta Derm. Venereol. 2012, 92, 241–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, M.A.; Bagatin, E. Skin barrier and microbiome in acne. Arch. Dermatol. Res. 2018, 310, 181–185. [Google Scholar] [CrossRef]
- Admyre, C.; Telemo, E.; Almqvist, N.; Lötvall, J.; Lahesmaa, R.; Scheynius, A.; Gabrielsson, S. Exosomes—Nanovesicles with possible roles in allergic inflammation. Allergy 2008, 63, 404–408. [Google Scholar] [CrossRef]
- Shin, T.S.; Kim, J.H.; Kim, Y.S.; Jeon, S.G.; Zhu, Z.; Gho, Y.S.; Kim, Y.K. Extracellular vesicles are key intercellular mediators in the development of immune dysfunction to allergens in the airways. Allergy 2010, 65, 1256–1265. [Google Scholar] [CrossRef] [Green Version]
- Kang, C.S.; Ban, M.; Choi, E.J.; Moon, H.G.; Jeon, J.S.; Kim, D.K.; Park, S.K.; Jeon, S.G.; Roh, T.Y.; Myung, S.J.; et al. Extracellular Vesicles Derived from Gut Microbiota, Especially Akkermansia muciniphila, Protect the Progression of Dextran Sulfate Sodium-Induced Colitis. PLoS ONE 2013, 8, e76520. [Google Scholar] [CrossRef] [Green Version]
- Volkova, L.; Khalif, I.; Kabanova, I. Impact of the impaired intestinal microflora on the course of acne vulgaris. Klin. Med. 2001, 79, 39–41. [Google Scholar]
- Wallace, T.C.; Guarner, F.; Madsen, K.; Cabana, M.D.; Gibson, G.; Hentges, E.; Sanders, M.E. Human gut microbiota and its relationship to health and disease. Nutr. Rev. 2011, 69, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, H.; Zhou, J.; Mou, Y.; Wang, G.; Xiong, X. Patients with acne vulgaris have a distinct gut microbiota in comparison with healthy controls. Acta Derm. Venereol. 2018, 98, 783–790. [Google Scholar] [CrossRef] [Green Version]
- Melnik, B.C. Acne vulgaris: The metabolic syndrome of the pilosebaceous follicle. Clin. Dermatol. 2018, 36, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Cong, T.X.; Hao, D.; Wen, X.; Li, X.H.; He, G.; Jiang, X. From pathogenesis of acne vulgaris to anti-acne agents. Arch. Dermatol. Res. 2019, 311, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.M.; Gilliland, K.; Clawson, G.A.; Thiboutot, D. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J. Investig. Dermatol. 2008, 128, 1286–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çerman, A.A.; Aktaş, E.; Altunay, I.K.; Arici, J.E.; Tulunay, A.; Ozturk, F.Y. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J. Am. Acad. Dermatol. 2016, 75, 155–162. [Google Scholar] [CrossRef]
- Lee, E.; Jung, S.R.; Lee, S.Y.; Lee, N.K.; Paik, H.D.; Lim, S. Il Lactobacillus plantarum strain ln4 attenuates diet-induced obesity, insulin resistance, and changes in hepatic mRNA levels associated with glucose and lipid metabolism. Nutrients 2018, 10, 643. [Google Scholar] [CrossRef] [Green Version]
- Bagarolli, R.A.; Tobar, N.; Oliveira, A.G.; Araújo, T.G.; Carvalho, B.M.; Rocha, G.Z.; Vecina, J.F.; Calisto, K.; Guadagnini, D.; Prada, P.O.; et al. Probiotics modulate gut microbiota and improve insulin sensitivity in DIO mice. J. Nutr. Biochem. 2017, 50, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Fabbrocini, G.; Bertona, M.; Picazo, O.; Pareja-Galeano, H.; Monfrecola, G.; Emanuele, E. Supplementation with Lactobacillus rhamnosus SP1 normalises skin expression of genes implicated in insulin signalling and improves adult acne. Benef. Microbes 2016, 7, 625–630. [Google Scholar] [CrossRef] [Green Version]
- Weichhart, T.; Säemann, M.D. The PI3K/Akt/mTOR pathway in innate immune cells: Emerging therapeutic applications. Ann. Rheum. Dis. 2008, 67, iii70–iii74. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.J.; Kim, S.H.; Han, J.; Bae, J.; Kim, S.J.; Park, C.G.; Chun, T. IL-10 inhibits the starvation induced autophagy in macrophages via class I phosphatidylinositol 3-kinase (PI3K) pathway. Mol. Immunol. 2011, 48, 720–727. [Google Scholar] [CrossRef]
- Su, X.; Yu, Y.; Zhong, Y.; Giannopoulou, E.G.; Hu, X.; Liu, H.; Cross, J.R.; Rätsch, G.; Rice, C.M.; Ivashkiv, L.B. Interferon-γ regulates cellular metabolism and mRNA translation to potentiate macrophage activation. Nat. Immunol. 2015, 16, 838–849. [Google Scholar] [CrossRef] [Green Version]
- Bowe, W.P.; Patel, N.B.; Logan, A.C. Acne vulgaris, probiotics and the gut-brain-skin axis: From anecdote to translational medicine. Benef. Microbes 2014, 5, 185–199. [Google Scholar] [CrossRef]
- Won, T.J.; Kim, B.; Lee, Y.; Bang, J.S.; Oh, E.S.; Yoo, J.S.; Hyung, K.E.; Yoon, J.; Hwang, S.; Park, E.S.; et al. Therapeutic potential of Lactobacillus plantarum CJLP133 for house-dust mite-induced dermatitis in NC/Nga mice. Cell. Immunol. 2012, 277, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Kwon, H.H.; Hong, J.S.; Yoon, J.Y.; Park, M.S.; Jang, M.Y.; Suh, D.H. Effect of dietary supplementation with omega-3 fatty acid and gamma-linolenic acid on acne vulgaris: A randomised, double-blind, controlled trial. Acta Derm. Venereol. 2014, 94, 521–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, A.A.; Jørgensen, L.G.M.; Nielsen, J.N.; Eivindson, M.; Grønbæk, H.; Vind, I.; Hougaardt, D.M.; Skogstrand, K.; Jensen, S.; Munkholm, P.; et al. Omega-3 fatty acids inhibit an increase of proinflammatory cytokines in patients with active Crohn’s disease compared with omega-6 fatty acids. Aliment. Pharmacol. Ther. 2005, 22, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Oláh, A.; Tóth, B.I.; Borbíró, I.; Sugawara, K.; Szöllõsi, A.G.; Czifra, G.; Pál, B.; Ambrus, L.; Kloepper, J.; Camera, E.; et al. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J. Clin. Investig. 2014, 124, 3713–3724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.; Kim, K.-P.; Ahn, H.Y.; Kim, B.; Cho, Y. Alterations in IL-4, IL-10 and IFN-γ levels synergistically decrease lipid content and protein expression of FAS and mature SREBP-1 in human sebocytes. Arch. Dermatol. Res. 2019, 311, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Peterson, T.R.; Sengupta, S.S.; Harris, T.E.; Carmack, A.E.; Kang, S.A.; Balderas, E.; Guertin, D.A.; Madden, K.L.; Carpenter, A.E.; Finck, B.N.; et al. mTOR complex 1 regulates lipin 1 localization to control the srebp pathway. Cell 2011, 146, 408–420. [Google Scholar] [CrossRef] [Green Version]
- Rosignoli, C.; Nicolas, J.C.; Jomard, A.; Michel, S. Involvement of the SREBP pathway in the mode of action of androgens in sebaceous glands in vivo. Exp. Dermatol. 2003, 12, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Hatano, Y.; Katagiri, K.; Arakawa, S.; Fujiwara, S. Interleukin-4 depresses levels of transcripts for acid-sphingomyelinase and glucocerebrosidase and the amount of ceramide in acetone-wounded epidermis, as demonstrated in a living skin equivalent. J. Dermatol. Sci. 2007, 47, 45–47. [Google Scholar] [CrossRef]




| Characteristics | CJLP55 Group | Placebo Group | p Value |
|---|---|---|---|
| (n = 14) | (n = 14) | ||
| Sex (male/female) | 7/7 | 5/9 | 0.445 2 |
| Age (years) | 24.29 ± 0.73 1 | 23.86 ± 0.80 | 0.695 3 |
| Body mass index (kg/m2) | 20.74 ± 0.64 | 21.39 ± 0.55 | 0.401 3 |
| Inflammatory lesion count (ILC) | 17.79 ± 3.19 | 19.64 ± 3.81 | 0.874 3 |
| Total lesion count (TLC) | 72.93 ± 10.11 | 98.14 ± 15.75 | 0.189 3 |
| Acne grade | 3.21 ± 0.19 | 3.14 ± 0.14 | 0.734 3 |
| CJLP55 Group (n = 14, 7 Male/7 female) | Placebo Group (n = 14, 5 Male/9 Female) | % Change between Groups (95%CI) | p Value 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Week12 | % Change | Baseline | Week12 | % Change | |||
| Inflammatory lesion count (ILC) | ||||||||
| Male subjects | 24.86 ± 4.74 | 6.71 ± 1.36 **1,2 | −72.99 ± 14.86 | 20.40 ± 6.78 | 15.40 ± 7.36 | −24.51 ± 15.88 | −48.48 (−97.84 to 0.89) 3 | 0.054 |
| Female subjects | 10.71 ± 2.24 | 4.57 ± 1.54 *2 | −57.33 ± 19.79 | 19.22 ± 4.89 | 14.00 ± 2.13 | −27.17 ± 16.31 | −30.17 (−84.67 to 24.34) | 0.255 |
| All subjects | 17.79 ± 3.19 | 5.64 ± 1.03 ***2 | −68.27 ± 14.83 | 19.64 ± 3.81 | 14.50 ± 2.79 *2 | −26.18 ± 11.43 | −42.09 (−80.57 to −3.61) | 0.033 |
| Total lesion count (TLC) | ||||||||
| Male subjects | 98.00 ± 13.73 | 39.57 ± 7.00 **2 | −59.62 ± 9.48 | 95.40 ± 28.62 | 78.00 ± 20.52 | −18.24 ± 10.83 | −41.38 (−73.67 to −9.10) | 0.017 |
| Female subjects | 47.86 ± 6.67 | 27.57 ± 6.15 **2 | −42.39 ± 10.41 | 99.67 ± 19.97 | 102.78 ± 20.02 | 3.12 ± 18.41 | −45.51 (−94.67 to 3.65) | 0.031 |
| All subjects | 72.93 ± 10.11 | 33.57 ± 4.77 ***2 | −53.97 ± 10.04 | 98.14 ± 15.75 | 93.93 ± 14.69 | −4.29 ± 12.58 | −49.67 (−82.76 to −16.59) | 0.002 |
| Acne grade | ||||||||
| Male subjects | 3.71 ± 0.18 | 2.86 ± 0.26 *2 | −23.08 ± 7.02 | 3.20 ± 0.20 | 3.40 ± 0.24 | 6.25 ± 6.25 | −29.33 (−51.36 to −7.29) | 0.030 |
| Female subjects | 2.71 ± 0.18 | 2.00 ± 0.31 *2 | −26.31 ± 6.79 | 3.11 ± 0.20 | 3.22 ± 0.15 | 3.57 ± 8.37 | −29.89 (−54.04 to −5.74) | 0.012 |
| All subjects | 3.21 ± 0.19 | 2.43 ± 0.23 **2 | −24.44 ± 4.81 | 3.14 ± 0.14 | 3.29 ± 0.13 | 4.55 ± 5.64 | −28.99 (−44.23 to −13.75) | 0.009 |
| CJLP55 Group (n = 14, 7 Male/7 Female) | Placebo Group (n = 14, 5 Male/9 Female) | % Change between Groups (95%CI) | p Value 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Week12 | % Change | Baseline | Week12 | % Change | |||
| Skin sebum (μg/cm2) | ||||||||
| All subjects | 178.82 ± 12.98 | 147.11 ± 16.40 *1,2 | −17.74 ± 6.04 | 202.36 ± 15.28 | 199.39 ± 16.44 | −1.47 ± 4.92 | −16.27 (−32.28 to −0.26) 3 | 0.005 |
| Skin hydration (capacitance in au.) | ||||||||
| All subjects | 73.47 ± 1.53 | 77.64 ± 1.99 | 5.67 ± 2.95 | 72.67 ± 1.73 | 66.24 ± 1.78 | −8.85 ± 2.58 | 14.52 (6.47 to 22.57) | 0.003 |
| Skin pH | ||||||||
| All subjects | 6.67 ± 0.15 | 5.40 ± 0.10 | −18.93 ± 2.70 | 6.69 ± 0.11 | 5.61 ± 0.11 | −16.17 ± 2.42 | −2.76 (−10.21 to 4.69) | 0.453 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-J.; Kim, K.-P.; Choi, E.; Yim, J.-H.; Choi, C.; Yun, H.-S.; Ahn, H.-Y.; Oh, J.-Y.; Cho, Y. Effects of Lactobacillus plantarum CJLP55 on Clinical Improvement, Skin Condition and Urine Bacterial Extracellular Vesicles in Patients with Acne Vulgaris: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2021, 13, 1368. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13041368
Kim M-J, Kim K-P, Choi E, Yim J-H, Choi C, Yun H-S, Ahn H-Y, Oh J-Y, Cho Y. Effects of Lactobacillus plantarum CJLP55 on Clinical Improvement, Skin Condition and Urine Bacterial Extracellular Vesicles in Patients with Acne Vulgaris: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 2021; 13(4):1368. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13041368
Chicago/Turabian StyleKim, Mi-Ju, Kun-Pyo Kim, Eunhye Choi, June-Hyuck Yim, Chunpil Choi, Hyun-Sun Yun, Hee-Yoon Ahn, Ji-Young Oh, and Yunhi Cho. 2021. "Effects of Lactobacillus plantarum CJLP55 on Clinical Improvement, Skin Condition and Urine Bacterial Extracellular Vesicles in Patients with Acne Vulgaris: A Randomized, Double-Blind, Placebo-Controlled Study" Nutrients 13, no. 4: 1368. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13041368