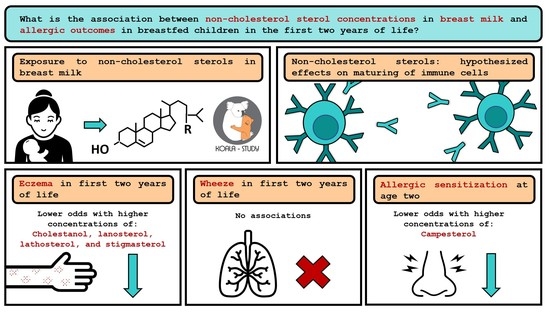
Non-Cholesterol Sterols in Breast Milk and Risk of Allergic Outcomes in the First Two Years of Life
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Outcomes
2.3. Breast Milk Sampling and Analysis of Non-Cholesterol Sterols
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Flow Chart
3.2. Selection Process of Sterols
3.2.1. Exploratory Factor Analysis
3.2.2. Multiple Logistic Regression Using the Factors
3.2.3. Independent-Sample t-Tests
3.3. Multiple Logistic Regression Using Selected Sterols
3.3.1. Eczema
3.3.2. Wheeze
3.3.3. Allergic Sensitization
3.4. Cholesterol and Allergic Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; França, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef] [Green Version]
- Infant and Young Child Feeding Home Page. Available online: https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding (accessed on 31 March 2021).
- Sankar, M.J.; Sinha, B.; Chowdhury, R.; Bhandari, N.; Taneja, S.; Martines, J.C.; Bahl, R. Optimal breastfeeding practices and infant and child mortality: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatrics 2017, 171, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.I.; Hwang, S.-J.; Ingelsson, E.; Benjamin, E.J.; Fox, C.S.; Vasan, R.S.; Murabito, J.M. Breastfeeding in Infancy and Adult Cardiovascular Disease Risk Factors. Am. J. Med. 2009, 122, 656–663.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umer, A.; Hamilton, C.; Edwards, R.A.; Cottrell, L.; Giacobbi, P.; Innes, K.; John, C.; Kelley, G.A.; Neal, W.; Lilly, C. Association between breastfeeding and childhood cardiovascular disease risk factors. Matern. Child Health J. 2019, 23, 228–239. [Google Scholar] [CrossRef]
- Horta, B.L.; Loret De Mola, C.; Victora, C.G. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 30–37. [Google Scholar] [CrossRef]
- Wang, L.; Collins, C.; Ratliff, M.; Xie, B.; Wang, Y. Breastfeeding Reduces Childhood Obesity Risks. Child. Obes. 2017, 13, 197–204. [Google Scholar] [CrossRef]
- Temples, H.S. Breastfeeding reduces risk of Type 2 Diabetes in the (PETS). Nurs. Outlook 2019, 67, 115. [Google Scholar] [CrossRef]
- Matheson, M.; Allen, K.; Tang, M. Understanding the evidence for and against the role of breastfeeding in allergy prevention. Clin. Exp. Allergy 2012, 42, 827–851. [Google Scholar] [CrossRef]
- Dogaru, C.M.; Nyffenegger, D.; Pescatore, A.M.; Spycher, B.D.; Kuehni, C.E. Breastfeeding and Childhood Asthma: Systematic Review and Meta-Analysis. Am. J. Epidemiol. 2014, 179, 1153–1167. [Google Scholar] [CrossRef] [Green Version]
- Klopp, A.; Vehling, L.; Becker, A.B.; Subbarao, P.; Mandhane, P.J.; Turvey, S.; Lefebvre, D.L.; Sears, M.R.; Azad, M.B.; Daley, D.; et al. Modes of Infant Feeding and the Risk of Childhood Asthma: A Prospective Birth Cohort Study. J. Pediatr. 2017, 190, 192–199.e2. [Google Scholar] [CrossRef] [PubMed]
- Lodge, C.J.; Tan, D.J.; Lau, M.X.Z.; Dai, X.; Tham, R.; Lowe, A.J.; Bowatte, G.; Allen, K.J.; Dharmage, S.C. Breastfeeding and asthma and allergies: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Kull, I.; Böhme, M.; Wahlgren, C.-F.; Nordvall, L.; Pershagen, G.; Wickman, M. Breast-feeding reduces the risk for childhood eczema. J. Allergy Clin. Immunol. 2005, 116, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Van Odijk, J.; Kull, I.; Borres, M.P.; Brandtzaeg, P.; Edberg, U.; Hanson, L.Å.; Høst, A.; Kuitunen, M.; Olsen, S.F.; Skerfving, S.; et al. Breastfeeding and allergic disease: A multidisciplinary review of the literature (1966–2001) on the mode of early feeding in infancy and its impact on later atopic manifestations. Allergy 2003, 58, 833–843. [Google Scholar] [CrossRef]
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast milk, a source of beneficial microbes and asso-ciated benefits for infant health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Nutritional Status During Pregnancy and Lactation. Nutrition During Lactation; (DC): National Academies Press: Washington, DC, USA, 1991. [Google Scholar]
- Prentice, P.; Ong, K.K.; Schoemaker, M.H.; Van Tol, E.A.F.; Vervoort, J.; Hughes, I.A.; Acerini, C.L.; Dunger, D.B. Breast milk nutrient content and infancy growth. Acta Paediatr. 2016, 105, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Jenness, R. The composition of human milk. Semin. Perinatol. 1979, 3, 225–239. [Google Scholar]
- Albrecht, C.; Huang, X.; Ontsouka, E. Cholesterol transporters in lactating and nonlactating human mammary tissue. In Handbook of Dietary and Nutritional Aspects of Human Breast Milk; Wageningen Academic Publishers: Wageningen, The Netherlands, 2013; p. e20895. [Google Scholar]
- Plat, J.; Baumgartner, S.; Vanmierlo, T.; Lütjohann, D.; Calkins, K.; Burrin, D.; Guthrie, G.; Thijs, C.; Velde, A.T.; Vreugdenhil, A.; et al. Plant-based sterols and stanols in health & disease: “Consequences of human development in a plant-based environment?”. Prog. Lipid Res. 2019, 74, 87–102. [Google Scholar] [CrossRef]
- Farke, C.; Viturro, E.; Meyer, H.H.D.; Albrecht, C. Identification of the bovine cholesterol efflux regulatory protein ABCA1 and its expression in various tissues1. J. Anim. Sci. 2006, 84, 2887–2894. [Google Scholar] [CrossRef]
- Ras, R.T.; Geleijnse, J.M.; Trautwein, E.A. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: A meta-analysis of randomised controlled studies. Br. J. Nutr. 2014, 112, 214–219. [Google Scholar] [CrossRef] [Green Version]
- de Jong, A.; Plat, J.; Mensink, R.P. Metabolic effects of plant sterols and stanols. J. Nutr. Biochem. 2003, 14, 362–369. [Google Scholar] [CrossRef]
- Brüll, F.; De Smet, E.; Mensink, R.P.; Vreugdenhil, A.; Kerksiek, A.; Lütjohann, D.; Wesseling, G.; Plat, J. Dietary plant stanol ester consumption improves immune function in asthma patients: Results of a randomized, double-blind clinical trial1. Am. J. Clin. Nutr. 2016, 103, 444–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brüll, F.; Mensink, R.P.; Steinbusch, M.F.; Husche, C.; Lütjohann, D.; Wesseling, G.J.; Plat, J. Beneficial effects of sito-stanol on the attenuated immune function in asthma patients: Results of an in vitro approach. PLoS ONE 2012, 7, e46895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plat, J.; Baumgartner, S.; Mensink, R.P. Mechanisms Underlying the Health Benefits of Plant Sterol and Stanol Ester Consumption. J. AOAC Int. 2015, 98, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.B. Allergy and allergic diseases. N. Engl. J. Med. 2001, 344, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Spann, N.J.; Garmire, L.X.; McDonald, J.G.; Myers, D.S.; Milne, S.B.; Shibata, N.; Reichart, D.; Fox, J.N.; Shaked, I.; Heudobler, D.; et al. Regulated Accumulation of Desmosterol Integrates Macrophage Lipid Metabolism and Inflammatory Responses. Cell 2012, 151, 138–152. [Google Scholar] [CrossRef] [Green Version]
- Bekkering, S.; Arts, R.; Novakovic, B.; Kourtzelis, I.; van der Heijden, C.; Li, Y.; Popa, C.; Ter Horst, R.; van Tuijl, J.; Netea-Maier, R.T.; et al. Metabolic Induction of Trained Immunity through the Mevalonate Pathway. Cell 2018, 172, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Miettinen, T.; Gylling, H.; Nissinen, M. The role of serum non-cholesterol sterols as surrogate markers of absolute cholesterol synthesis and absorption. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 765–769. [Google Scholar] [CrossRef]
- Kummeling, I.; Thijs, C.; Penders, J.; Snijders, B.E.P.; Stelma, F.; Reimerink, J.; Koopmans, M.; Dagnelie, P.C.; Huber, M.; Jansen, M.C.J.F.; et al. Etiology of atopy in infancy: The KOALA Birth Cohort Study. Pediatr. Allergy Immunol. 2005, 16, 679–684. [Google Scholar] [CrossRef]
- Ygberg, S.; Nilsson, A. The developing immune system—From foetus to toddler. Acta Paediatr. 2012, 101, 120–127. [Google Scholar] [CrossRef]
- Thijs, C.; Müller, A.; Rist, L.; Kummeling, I.; Snijders, B.; Huber, M.; Van Ree, R.; Simões-Wüst, A.; Dagnelie, P.; Van Den Brandt, P. Fatty acids in breast milk and development of atopic eczema and allergic sensitisation in infancy. Allergy 2011, 66, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Akkerdaas, J.H.; Wensing, M.; Asero, R.; Rivas, M.F.; Knulst, A.C.; Bolhaar, S.; Hefle, S.L.; Aalberse, R.C.; Van Ree, R. IgE Binding to Pepsin-Digested Food Extracts. Int. Arch. Allergy Immunol. 2005, 138, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Kummeling, I.; Thijs, C.; Huber, M.; van de Vijver, L.P.L.; Snijders, B.E.P.; Penders, J.; Stelma, F.; van Ree, R.; Brandt, P.A.V.D.; Dagnelie, P.C. Consumption of organic foods and risk of atopic disease during the first 2 years of life in the Netherlands. Br. J. Nutr. 2008, 99, 598–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rist, L.; Mueller, A.; Barthel, C.; Snijders, B.; Jansen, M.; Simões-Wüst, A.P.; Huber, M.; Kummeling, I.; von Mandach, U.; Steinhart, H.; et al. Influence of organic diet on the amount of conjugated linoleic acids in breast milk of lactating women in the Netherlands. Br. J. Nutr. 2007, 97, 735–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, A.; A Gibbs, J.; Lyster, R.L.; Baum, J.D. Creamatocrit: Simple clinical technique for estimating fat concentration and energy value of human milk. BMJ 1978, 1, 1018–1020. [Google Scholar] [CrossRef] [Green Version]
- Chan, M.K.; Huang, Y.C.; Varghese, Z.; Baillod, R.A.; Moorhead, J.F. Predicting plasma concentrations of common biochemical values from residual peritoneal fluid in patients on peritoneal dialysis. Br. Med. J. 1978, 1, 1670. [Google Scholar] [CrossRef] [Green Version]
- Thelen, K.M.; Laaksonen, R.; Päivä, H.; Lehtimäki, T.; Lütjohann, D. High-Dose Statin Treatment Does Not Alter Plasma Marker for Brain Cholesterol Metabolism in Patients with Moderately Elevated Plasma Cholesterol Levels. J. Clin. Pharmacol. 2006, 46, 812–816. [Google Scholar] [CrossRef]
- Ferrante, G.; Carta, M.; Montante, C.; Notarbartolo, V.; Corsello, G.; Giuffrè, M. Current Insights on Early Life Nutrition and Prevention of Allergy. Front. Pediatrics 2020, 8, 448. [Google Scholar] [CrossRef]
- Alwarith, J.; Kahleova, H.; Crosby, L.; Brooks, A.; Brandon, L.; Levin, S.M.; Barnard, N.D. The role of nutrition in asthma prevention and treatment. Nutr. Rev. 2020, 78, 928–938. [Google Scholar] [CrossRef] [Green Version]
- Cepeda, A.M.; The ISAAC Phase III Latin America Group; Thawer, S.; Boyle, R.J.; Villalba, S.; Jaller, R.; Tapias, E.; Segura, A.M.; Villegas, R.; Larsen, V.G.; et al. Diet and Respiratory Health in Children from 11 Latin American Countries: Evidence from ISAAC Phase III. Lung 2017, 195, 683–692. [Google Scholar] [CrossRef] [Green Version]
- van Gorp, C.; de Lange, I.H.; Spiller, O.B.; Dewez, F.; Cillero, P.B.; Heeren, R.; Kessels, L.; Kloosterboer, N.; van Gemert, W.G.; Beeton, M.L. Protection of the ovine fetal gut against ureaplasma-induced chorioamnionitis: A potential role for plant sterols. Nutrients 2019, 11, 968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Smet, E.; Mensink, R.P.; Boekschoten, M.V.; de Ridder, R.; Germeraad, W.T.; Wolfs, T.G.; Plat, J. An acute intake of plant stanol esters alters immune-related pathways in the jejunum of healthy volunteers. Br. J. Nutr. 2015, 113, 794–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claumarchirant, L.; Matencio, E.; Sanchez-Siles, L.M.; Alegría, A.; Lagarda, M.J. Sterol composition in infant formulas and estimated intake. J. Agric. Food Chem. 2015, 63, 7245–7251. [Google Scholar] [CrossRef] [PubMed]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W.; Committee on Nutrition; Section on Allergy and Immunology. The Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Hydrolyzed Formulas, and Timing of Introduction of Allergenic Complementary Foods. Pediatrics 2019, 143, e20190281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laubereau, B.; Brockow, I.; Zirngibl, A.; Koletzko, S.; Gruebl, A.; von Berg, A.; Filipiak-Pittroff, B.; Berdel, D.; Bauer, C.P.; Reinhardt, D. Effect of breast-feeding on the development of atopic dermatitis during the first 3 years of life—Results from the GINI-birth cohort study. J. Pediatrics 2004, 144, 602–607. [Google Scholar] [CrossRef]
- Friedman, N.J.; Zeiger, R. The role of breast-feeding in the development of allergies and asthma. J. Allergy Clin. Immunol. 2005, 115, 1238–1248. [Google Scholar] [CrossRef]
- Woollett, L.A.; Heubi, J.E. Fetal and Neonatal Cholesterol Metabolism; MDText.com, Inc.: South Dartmouth, MA, USA, 2016. [Google Scholar]
- Delplanque, B.; Gibson, R.; Koletzko, B.; Lapillonne, A.; Strandvik, B. Lipid quality in infant nutrition: Current knowledge and future opportunities. J. Pediatric Gastroenterol. Nutr. 2015, 61, 8. [Google Scholar] [CrossRef] [Green Version]
- Le Doare, K.; Holder, B.; Bassett, A.; Pannaraj, P.S. Mother’s milk: A purposeful contribution to the development of the infant microbiota and immunity. Front. Immunol. 2018, 9, 361. [Google Scholar] [CrossRef] [Green Version]
- Henderson, J.; Granell, R.; Heron, J.; Sherriff, A.; Simpson, A.; Woodcock, A.; Strachan, D.P.; Shaheen, S.O.; Sterne, J.A. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax 2008, 63, 974–980. [Google Scholar] [CrossRef] [Green Version]
- Granell, R.; Sterne, J.A.; Henderson, J. Associations of different phenotypes of wheezing illness in early childhood with environmental variables implicated in the aetiology of asthma. PLoS ONE 2012, 7, e48359. [Google Scholar] [CrossRef] [Green Version]

| Total (N = 311) | Conventional Lifestyle 1 (N = 141) | Alternative Lifestyle 1 (N = 166) | |
|---|---|---|---|
| Maternal age, years (SD) | 32.4 (3.9) | 31.5 (3.4) | 33.1 (4.2) |
| Prepregnancy BMI, kg/m2 (IQR) | 22.4 (20.6–24.5) | 23.0 (21.5–25.2) | 21.7 (20.1–24.0) |
| Smoking during pregnancy 2, N (%) | 5 (2%) | 4 (3%) | 1 (1%) |
| Atopic history parents 1, N (%) | |||
| None | 113 (37%) | 50 (36%) | 62 (38%) |
| Only father | 78 (25%) | 37 (26%) | 40 (24%) |
| Only mother | 64 (21%) | 31 (22%) | 32 (20%) |
| Both | 52 (17%) | 22 (16%) | 30 (18%) |
| Gender child female, N (%) | 161 (52%) | 74 (53%) | 83 (50%) |
| Duration breastfeeding, N (%) | |||
| 1–3 months | 64 (21%) | 46 (33%) | 17 (10%) |
| 4–6 months | 70 (23%) | 40 (28%) | 29 (18%) |
| 7–9 months | 70 (23%) | 28 (20%) | 41 (25%) |
| 10–12 months | 53 (17%) | 17 (12%) | 36 (22%) |
| ≥13 months | 53 (17%) | 10 (7%) | 43 (26%) |
| Maternal education, N (%) | |||
| Lower | 12 (4%) | 7 (5%) | 5 (3%) |
| Middle | 96 (31%) | 53 (38%) | 41 (25%) |
| Higher vocational | 131 (42%) | 60 (43%) | 69 (42%) |
| Academic | 66 (21%) | 19 (14%) | 47 (28%) |
| Other | 6 (2%) | 2 (1%) | 4 (2%) |
| Season breast milk sampling 1, N (%) | |||
| December 2002–February 2003 | 112 (37%) | 64 (45%) | 48 (29%) |
| March–May 2003 | 120 (39%) | 59 (42%) | 61 (37%) |
| June–September 2003 | 75 (24%) | 18 (13%) | 57 (34%) |
| Gravidity, N (%) | |||
| 1 | 123 (40%) | 65 (46%) | 58 (34.9) |
| 2 | 110 (35%) | 51 (36%) | 57 (34.3) |
| ≥3 | 78 (25%) | 25 (18%) | 51 (30.7) |
| Eczema in first two years 3, N (%) | 91 (30%) | 41 (30%) | 50 (31.1) |
| Wheeze in first two years 4, N (%) | 79 (26%) | 37 (27%) | 42 (26%) |
| Allergic sensitization at age 2 5, N (%) | 49 (24%) | 25 (28%) | 24 (22%) |
| Creamatocrit value 6, % (IQR) | 7 (5–9%) | 7 (5–9%) | 7 (5–9%) |
| Cholesterol concentration breast milk, mmol/L (IQR) | 0.35 (0.28–0.42) | 0.35 (0.27–0.43) | 0.36 (0.29–0.41) |
| Cholesterol concentration breast milk corrected for creamatocrit 7, mmol/Lf (IQR) | 4.81 (4.14–5.90) | 4.81 (4.17–5.93) | 4.83 (5.84–6.87) |
| Non-cholesterol sterol concentrations breast milk, µmol/L (IQR) | |||
| Brassicasterol | 0.23 (0.18–0.27) | 0.24 (0.19–0.29) | 0.22 (0.17–0.25) |
| Campesterol 7 | 0.32 (0.20–0.52) | 0.37 (0.21–0.60) | 0.28 (0.19–0.46) |
| Cholestanol | 1.51 (1.28–1.73) | 1.50 (1.26–1.75) | 1.52 (1.31–1.73) |
| Desmosterol | 52.2 (37.4–70.3) | 51.7 (36.3–68.3) | 54.3 (38.1–71.8) |
| Lanosterol | 2.03 (1.43–2.89) | 2.01 (1.31–2.85) | 2.04 (1.50–2.93) |
| Lathosterol | 0.62 (0.40–0.84) | 0.62 (0.38–0.87) | 0.62 (0.40–0.84) |
| Sitosterol | 0.70 (0.49–1.41) | 0.59 (0.45–0.87) | 0.82 (0.55–1.48) |
| Stigmasterol | 0.05 (0.04–0.06) | 0.05 (0.04–0.06) | 0.05 (0.04–0.06) |
| Non-cholesterol sterol concentrations breast milk corrected for creamatocrit, µmol/Lf (IQR) | |||
| Brassicasterol 7 | 3.18 (2.44–4.16) | 3.32 (2.53–4.25) | 2.95 (2.31–4.10) |
| Campesterol 8 | 4.90 (3.01–7.07) | 5.34 (3.50–7.87) | 4.27 (2.61–6.13) |
| Cholestanol 7 | 21.0 (17.2–27.7) | 22.5 (17.3–27.9) | 20.5 (17.0–27.9) |
| Desmosterol 7 | 776.5 (592.7–997.9) | 716.1(561.6–957.0) | 807.2 (633.6–1026.5) |
| Lanosterol 7 | 29.1 (22.7–37.5) | 28.4 (22.8–37.3) | 29.7 (22.7–37.5) |
| Lathosterol 7 | 8.69 (6.25–11.9) | 8.80 (6.47–12.2) | 8.31 (5.99–11.6) |
| Sitosterol 7 | 10.3 (7.17–18.7) | 8.26 (6.60–12.6) | 13.7 (7.88–23.8) |
| Stigmasterol 7 | 0.68 (0.51–0.93) | 0.67 (0.51–0.93) | 0.69 (0.51–0.94) |
| Non-Cholesterol Sterol (µmol/Lf) | Factor 1 | Factor 2 |
|---|---|---|
| Cholestanol | 0.88 | 0.34 |
| Brassicasterol | 0.83 | |
| Stigmasterol | 0.78 | |
| Sitosterol | 0.65 | 0.31 |
| Campesterol | 0.64 | |
| Lathosterol | 0.37 | |
| Lanosterol | 0.93 | |
| Desmosterol | 0.85 |
| Factor 1 | Factor 2 | |||||
|---|---|---|---|---|---|---|
| Outcome Variable | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| Eczema (N = 256) 1 | 0.69 | 0.46; 1.03 | 0.07 * | 0.69 | 0.46; 1.04 | 0.08 * |
| Wheeze (N = 259) 2 | 1.04 | 0.77; 1.40 | 0.82 | 0.99 | 0.69; 1.42 | 0.95 |
| Allergic sensitization (N = 171) 3 | 0.52 | 0.26; 1.07 | 0.07 * | 1.17 | 0.79; 1.73 | 0.43 |
| Eczema | Wheeze | Allergic Sensitization | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sterol (µmol/Lf) | Mean Difference | 95% CI | p-Value | Mean Difference | 95% CI | p-Value | Mean Difference | 95% CI | p-Value |
| Brassicasterol | −0.11 | −0.76; 0.55 | 0.75 | 0.16 | −0.52; 0.84 | 0.64 | −0.25 | −1.06; 0.55 | 0.54 |
| Campesterol | −0.76 | −1.85; 0.32 | 0.17 | 0.11 | −1.03; 1.26 | 0.84 | −1.78 | −3.40; −0.16 | 0.03 ** |
| Cholestanol | −3.19 | −7.33; 0.94 | 0.13 | 0.3 | −4.08; 4.67 | 0.89 | −2.68 | −8.04; 2.68 | 0.33 |
| Desmosterol 1 | −0.04 | −0.14; 0.06 | 0.44 | 0.01 | −0.11; 0.13 | 0.83 | −0.05 | −0.22; 0.12 | 0.57 |
| Lanosterol | −2.03 | −5.55; 1.48 | 0.26 | −0.36 | −4.82; 4.10 | 0.88 | 0.94 | −4.77; 6.65 | 0.75 |
| Lathosterol | −1.18 | −2.39; 0.03 | 0.06 * | −0.93 | −2.23; 0.36 | 0.16 | −0.53 | −2.22; 1.16 | 0.54 |
| Sitosterol | −0.13 | −0.28; 0.03 | 0.11 | −0.1 | −0.26; 0.07 | 0.25 | −0.13 | −0.31; 0.04 | 0.14 |
| Stigmasterol | −0.01 | −0.02; 0.00 | 0.08 * | 0 | −0.01; 0.01 | 0.89 | −0.01 | −0.02; 0.01 | 0.36 |
| Sterol (µmol/Lf) | N | OR | 95% CI | p-Value |
|---|---|---|---|---|
| Brassicasterol 1 | 264 | 0.95 | 0.87; 1.03 | 0.22 |
| Campesterol 2 | 259 | 0.95 | 0.88; 1.03 | 0.25 |
| Cholestanol 3 | 264 | 0.98 | 0.95; 1.00 | 0.04 * |
| Desmosterol a,1 | 264 | 0.52 | 0.22; 1.22 | 0.13 |
| Lanosterol 4 | 267 | 0.97 | 0.95; 1.00 | 0.02 * |
| Lathosterol 5 | 267 | 0.93 | 0.87; 0.99 | 0.02 * |
| Sitosterol 6 | 264 | 0.98 | 0.95; 1.00 | 0.09 |
| Stigmasterol 6 | 264 | 0.51 | 0.29; 0.91 | 0.02 * |
| Sterol (µmol/Lf) | N | OR | 95% CI | p-Value |
|---|---|---|---|---|
| Brassicasterol 1 | 176 | 0.93 | 0.78; 1.12 | 0.47 |
| Campesterol 2 | 171 | 0.81 | 0.70; 0.95 | 0.01 * |
| Cholestanol 3 | 176 | 0.98 | 0.95; 1.01 | 0.26 |
| Lathosterol 4 | 176 | 0.99 | 0.91; 1.07 | 0.77 |
| Sitosterol 5 | 176 | 0.97 | 0.93; 1.01 | 0.13 |
| Stigmasterol 5 | 176 | 0.77 | 0.42; 1.40 | 0.38 |
| Sterol (µmol/Lf) | Spearman’s ρ Cholesterol (mmol/Lf) |
|---|---|
| Brassicasterol | −0.42 ** |
| Campesterol 1 | 0.40 ** |
| Cholestanol | −0.45 ** |
| Desmosterol | −0.13 * |
| Lanosterol | −0.07 |
| Lathosterol | −0.04 |
| Sitosterol | −0.37 ** |
| Stigmasterol | −0.51 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Brakel, L.; Thijs, C.; Mensink, R.P.; Lütjohann, D.; Plat, J. Non-Cholesterol Sterols in Breast Milk and Risk of Allergic Outcomes in the First Two Years of Life. Nutrients 2022, 14, 766. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14040766
van Brakel L, Thijs C, Mensink RP, Lütjohann D, Plat J. Non-Cholesterol Sterols in Breast Milk and Risk of Allergic Outcomes in the First Two Years of Life. Nutrients. 2022; 14(4):766. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14040766
Chicago/Turabian Stylevan Brakel, Lieve, Carel Thijs, Ronald P. Mensink, Dieter Lütjohann, and Jogchum Plat. 2022. "Non-Cholesterol Sterols in Breast Milk and Risk of Allergic Outcomes in the First Two Years of Life" Nutrients 14, no. 4: 766. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14040766