Vitamin B Complex and Experimental Autoimmune Encephalomyelitis –Attenuation of the Clinical Signs and Gut Microbiota Dysbiosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
2.3. Induction and Treatment of Experimental Autoimmune Encephalomyelitis (EAE)
2.4. Clinical Assessment of EAE
2.5. The Determination of Nerve/Muscle Nuclear Density and Histological Examination of Popliteal Lymph Nodes
2.6. Tissue Collection and DNA Extraction for 16S rRNA GENE Sequencing
2.7. 16S rRNA Protocol
2.8. Statistical Analysis
3. Results
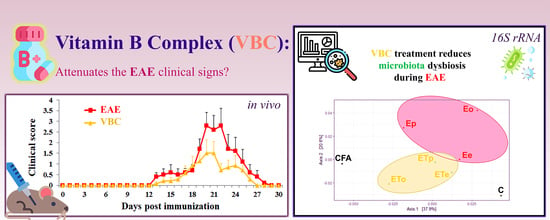
3.1. VBC Treatment Attenuates Severity and Duration of EAE
3.2. Histological Examination of Popliteal Lymph Nodes as Drain Lymph Nodes after Induction of EAE
3.3. Nerve Nuclear Density
3.4. Muscle Nuclear Density
3.5. Gut Microbiota Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palumbo, S.; Pellegrini, S. Experimental in vivo models of multiple sclerosis: State of the art. Exon Publ. 2017, 2017, 173–183. [Google Scholar]
- Bert, A.; Gran, B.; Weissert, R. EAE: Imperfect but useful models of multiple sclerosis. Trends Mol. Med. 2011, 17, 119–125. [Google Scholar]
- Bjelobaba, I.; Begovic-Kupresanin, V.; Pekovic, S.; Lavrnja, I. Animal models of multiple sclerosis: Focus on experimental autoimmune encephalomyelitis. J. Neurosci. Res. 2018, 96, 1021–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantinescu, C.S.; Farooqi, N.; O’Brien, K.; Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef] [PubMed]
- Bjelobaba, I.; Savic, D.; Lavrnja, I. Multiple sclerosis and neuroinflammation: The overview of current and prospective therapies. Curr. Pharm. Des. 2017, 23, 693–730. [Google Scholar] [CrossRef] [PubMed]
- Gold, R.; Hartung, H.-P.; Toyka, K.V. Animal models for autoimmune demyelinating disorders of the nervous system. Mol. Med. Today 2000, 6, 88–91. [Google Scholar] [CrossRef]
- Gold, R.; Linington, C.; Lassmann, H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain 2006, 129, 1953–1971. [Google Scholar] [CrossRef]
- Robinson, A.P.; Harp, C.T.; Noronha, A.; Miller, S.D. The experimental autoimmune encephalomyelitis (EAE) model of MS: Utility for understanding disease pathophysiology and treatment. Handb. Clin. Neurol. 2014, 122, 173–189. [Google Scholar]
- Lee, M.J.; Jang, M.; Choi, J.; Lee, G.; Min, H.J.; Chung, W.-S.; Kim, J.-I.; Jee, Y.; Chae, Y.; Kim, S.-H. Bee venom acupuncture alleviates experimental autoimmune encephalomyelitis by upregulating regulatory T cells and suppressing Th1 and Th17 responses. Mol. Neurobiol. 2016, 53, 1419–1445. [Google Scholar] [CrossRef]
- McLean, M.H.; Dieguez, D.; Miller, L.M.; Young, H.A. Does the microbiota play a role in the pathogenesis of autoimmune diseases? Gut 2015, 64, 332–341. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [Green Version]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, ra158–ra263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jangi, S.; Gandhi, R.; Cox, L.M.; Li, N.; Von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.L. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef] [Green Version]
- Johanson, D.M.; Goertz, J.E.; Marin, I.A.; Costello, J.; Overall, C.C.; Gaultier, A. Experimental autoimmune encephalomyelitis is associated with changes of the microbiota composition in the gastrointestinal tract. Sci. Rep. 2020, 10, 15183. [Google Scholar] [CrossRef] [PubMed]
- van den Hoogen, W.J.; Laman, J.D.; t Hart, B.A. Modulation of multiple sclerosis and its animal model experimental autoimmune encephalomyelitis by food and gut microbiota. Front. Immunol. 2017, 8, 1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.K.; Menezes, J.S.; Umesaki, Y.; Mazmanian, S.K. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2011, 108, 4615–4622. [Google Scholar] [CrossRef] [Green Version]
- Ntranos, A.; Park, H.-J.; Wentling, M.; Tolstikov, V.; Amatruda, M.; Inbar, B.; Kim-Schulze, S.; Frazier, C.; Button, J.; Kiebish, M.A. Bacterial neurotoxic metabolites in multiple sclerosis cerebrospinal fluid and plasma. Brain 2021, awab320. [Google Scholar] [CrossRef] [PubMed]
- Berer, K.; Mues, M.; Koutrolos, M.; Rasbi, Z.A.; Boziki, M.; Johner, C.; Wekerle, H.; Krishnamoorthy, G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011, 479, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Altun, I.; Kurutaş, E.B. Vitamin B complex and vitamin B12 levels after peripheral nerve injury. Neural Regen. Res. 2016, 11, 842. [Google Scholar] [CrossRef] [PubMed]
- Nemazannikova, N.; Mikkelsen, K.; Stojanovska, L.; Blatch, G.L.; Apostolopoulos, V. Is there a link between vitamin B and multiple sclerosis? Med. Chem. 2018, 14, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Nedeljković, P.; Dacić, S.; Kovačević, M.; Peković, S.; Vučević, D.; Božić-Nedeljković, B. Vitamin B complex as a potential therapeutical modality in combating peripheral nerve injury. Acta Med. Median. 2018, 57, 85–91. [Google Scholar] [CrossRef]
- Baltrusch, S. The Role of neurotropic B vitamins in nerve regeneration. BioMed Res. Int. 2021, 2021, 9968228. [Google Scholar] [CrossRef] [PubMed]
- El Soury, M.; Fornasari, B.E.; Carta, G.; Zen, F.; Haastert-Talini, K.; Ronchi, G. The role of dietary nutrients in peripheral nerve regeneration. Int. J. Mol. Sci. 2021, 22, 7417. [Google Scholar] [CrossRef] [PubMed]
- Nedeljković, P.; Zmijanjac, D.; Drašković-Pavlović, B.; Vasiljevska, M.; Vučević, D.; Božić, B.; Bumbaširević, M. Vitamin B complex treatment improves motor nerve regeneration and recovery of muscle function in a rodent model of peripheral nerve injury. Arch. Biol. Sci. 2017, 69, 361–368. [Google Scholar] [CrossRef]
- Ehmedah, A.; Nedeljkovic, P.; Dacic, S.; Repac, J.; Draskovic-Pavlovic, B.; Vučević, D.; Pekovic, S.; Nedeljkovic, B.B. Effect of vitamin B complex treatment on macrophages to schwann cells association during neuroinflammation after peripheral nerve injury. Molecules 2020, 25, 5426. [Google Scholar] [CrossRef] [PubMed]
- Ehmedah, A.; Nedeljkovic, P.; Dacic, S.; Repac, J.; Draskovic Pavlovic, B.; Vucevic, D.; Pekovic, S.; Bozic Nedeljkovic, B. Vitamin B complex treatment attenuates local inflammation after peripheral nerve injury. Molecules 2019, 24, 4615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeBlanc, J.G.; Milani, C.; De Giori, G.S.; Sesma, F.; Van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing [Internet]; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef] [Green Version]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. microeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.; Stojanovska, L.; Tangalakis, K.; Bosevski, M.; Apostolopoulos, V. Cognitive decline: A vitamin B perspective. Maturitas 2016, 93, 108–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leblhuber, F.; Ehrlich, D.; Steiner, K.; Geisler, S.; Fuchs, D.; Lanser, L.; Kurz, K. The immunopathogenesis of Alzheimer’s disease is related to the composition of gut microbiota. Nutrients 2021, 13, 361. [Google Scholar] [CrossRef]
- Reinhold, A.; Rittner, H. Barrier function in the peripheral and central nervous system—A review. Pflügers Arch. Eur. J. Physiol. 2017, 469, 123–134. [Google Scholar] [CrossRef]
- Castro, F.R.; Farias, A.S.; Proença, P.L.; de La Hoz, C.; Langone, F.; Oliveira, E.C.; Toyama, M.H.; Marangoni, S.; Santos, L.M. The effect of treatment with crotapotin on the evolution of experimental autoimmune neuritis induced in Lewis rats. Toxicon 2007, 49, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Nazareth, L.; St John, J.; Murtaza, M.; Ekberg, J. Phagocytosis by peripheral glia: Importance for nervous system functions and implications in injury and disease. Front. Cell Dev. Biol. 2021, 9, 660259. [Google Scholar] [CrossRef]
- Lee, S.K.; Wolfe, S.W. Peripheral nerve injury and repair. J. Am. Acad. Orthop. Surg. 2000, 8, 243–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Peng, J.; Han, G.-H.; Ding, X.; Wei, S.; Gao, G.; Huang, K.; Chang, F.; Wang, Y. Role of macrophages in peripheral nerve injury and repair. Neural Regen. Res. 2019, 14, 1335. [Google Scholar] [PubMed]
- Miller, A.; Korem, M.; Almog, R.; Galboiz, Y. Vitamin B12, demyelination, remyelination and repair in multiple sclerosis. J. Neurol. Sci. 2005, 233, 93–97. [Google Scholar] [CrossRef]
- Dardiotis, E.; Arseniou, S.; Sokratous, M.; Tsouris, Z.; Siokas, V.; Mentis, A.-F.A.; Michalopoulou, A.; Andravizou, A.; Dastamani, M.; Paterakis, K. Vitamin B12, folate, and homocysteine levels and multiple sclerosis: A meta-analysis. Mult. Scler. Relat. Disord. 2017, 17, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Mallone, F.; Lucchino, L.; Franzone, F.; Marenco, M.; Carlesimo, S.C.; Moramarco, A. High-dose vitamin B supplementation for persistent visual deficit in multiple sclerosis: A pilot study. Drug Discov. Ther. 2020, 14, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Borisov, A.B.; Dedkov, E.I.; Carlson, B.M. Interrelations of myogenic response, progressive atrophy of muscle fibers, and cell death in denervated skeletal muscle. Anat. Rec. Off. Publ. Am. Assoc. Anat. 2001, 264, 203–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, B.S. Role of the gut microbiota in human nutrition and metabolism. J. Gastroenterol. Hepatol. 2013, 28, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Uebanso, T.; Shimohata, T.; Mawatari, K.; Takahashi, A. Functional Roles of B-Vitamins in the Gut and Gut Microbiome. Mol. Nutr. Food Res. 2020, 64, 2000426. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.K.; Freedman, S.N.; Murra, A.C.; Zarei, K.; Sompallae, R.; Gibson-Corley, K.N.; Karandikar, N.J.; Murray, J.A.; Mangalam, A.K. Prevotella histicola, a human gut commensal, is as potent as COPAXONE® in an animal model of multiple sclerosis. Front. Immunol. 2019, 462. [Google Scholar] [CrossRef] [Green Version]
- Schepici, G.; Silvestro, S.; Bramanti, P.; Mazzon, E. The gut microbiota in multiple sclerosis: An overview of clinical trials. Cell Transplant. 2019, 28, 1507–1527. [Google Scholar] [CrossRef] [Green Version]
- Maghzi, A.-H.; Weiner, H.L. A One-Two Punch in the Gut May Trigger Multiple Sclerosis. Immunity 2020, 53, 707–709. [Google Scholar] [CrossRef]
- Zheng, Z.; Lyu, W.; Ren, Y.; Li, X.; Zhao, S.; Yang, H.; Xiao, Y. Allobaculum Involves in the Modulation of Intestinal ANGPTLT4 Expression in Mice Treated by High-Fat Diet. Front. Nutr. 2021, 8, 242. [Google Scholar] [CrossRef]
- Kaakoush, N.O. Insights into the role of Erysipelotrichaceae in the human host. Front. Cell. Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saresella, M.; Mendozzi, L.; Rossi, V.; Mazzali, F.; Piancone, F.; LaRosa, F.; Marventano, I.; Caputo, D.; Felis, G.E.; Clerici, M. Immunological and clinical effect of diet modulation of the gut microbiome in multiple sclerosis patients: A pilot study. Front. Immunol. 2017, 8, 1391. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandić, M.; Mitić, K.; Nedeljković, P.; Perić, M.; Božić, B.; Lunić, T.; Bačić, A.; Rajilić-Stojanović, M.; Peković, S.; Božić Nedeljković, B. Vitamin B Complex and Experimental Autoimmune Encephalomyelitis –Attenuation of the Clinical Signs and Gut Microbiota Dysbiosis. Nutrients 2022, 14, 1273. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14061273
Mandić M, Mitić K, Nedeljković P, Perić M, Božić B, Lunić T, Bačić A, Rajilić-Stojanović M, Peković S, Božić Nedeljković B. Vitamin B Complex and Experimental Autoimmune Encephalomyelitis –Attenuation of the Clinical Signs and Gut Microbiota Dysbiosis. Nutrients. 2022; 14(6):1273. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14061273
Chicago/Turabian StyleMandić, Marija, Katarina Mitić, Predrag Nedeljković, Mina Perić, Bojan Božić, Tanja Lunić, Ana Bačić, Mirjana Rajilić-Stojanović, Sanja Peković, and Biljana Božić Nedeljković. 2022. "Vitamin B Complex and Experimental Autoimmune Encephalomyelitis –Attenuation of the Clinical Signs and Gut Microbiota Dysbiosis" Nutrients 14, no. 6: 1273. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14061273