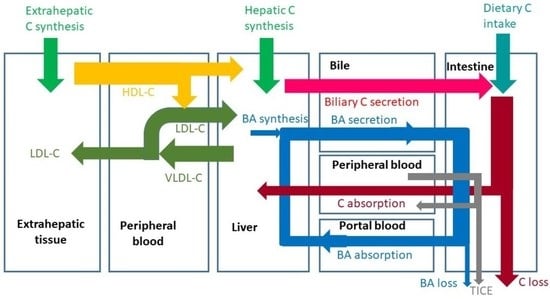
From Dietary Cholesterol to Blood Cholesterol, Physiological Lipid Fluxes, and Cholesterol Homeostasis
Abstract
:1. Introduction
2. Dietary C Intake
3. Known and Unknown C Fluxes in Humans
4. Fat Digestion and Intestinal Transport
5. Absorption
6. Hepatic C Metabolism
7. Biliary C Secretion and Trans-Intestinal C Excretion (TICE)
8. Lipoprotein Metabolism
9. C Flux Measurements in Humans
10. Discussion
- C intake;
- Digestion including gastric function, pancreatic function;
- Hepatic BA synthesis and biliary secretion;
- Biliary C secretion (NPC1-L1, ABCG5/G8);
- Cholecystokinin (CCK) production and release;
- Gallbladder function (contraction in response to CCK, relaxation);
- Intestinal micelle formation, micellar uptake of C;
- C uptake into enterocytes (NPC1-L1) and re-secretion into intestinal lumen (ABCG5/G8);
- TICE;
- CM formation, release and conversion to CMRs.
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nijstad, N.; Gautier, T.; Briand, F.; Rader, D.J.; Tietge, U.J. Biliary Sterol Secretion Is Required for Functional In Vivo Reverse Cholesterol Transport in Mice. Gastroenterology 2011, 140, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.F.; Rader, D.J. New Insights into the Regulation of HDL Metabolism and Reverse Cholesterol Transport. Circ. Res. 2005, 24, 1221–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, M.D.; Rudel, L.L. Review of cholesterol absorption with emphasis on dietary and biliary cholesterol. J. Lipid Res. 1994, 35, 943–955. [Google Scholar] [CrossRef]
- Lütjohann, D.; Meyer, S.; Von Bergmann, K.; Stellaard, F. Cholesterol Absorption and Synthesis in Vegetarians and Omnivores. Mol. Nutr. Food Res. 2018, 62, e1700689. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-F.; Yang, J.-J.; Lipworth, L.; Shu, X.-O.; Cai, H.; Steinwandel, M.; Blot, W.; Zheng, W.; Yu, D. Cholesterol and Egg Intakes with Cardiometabolic and All-Cause Mortality among Chinese and Low-Income Black and White Americans. Nutrients 2021, 13, 2094. [Google Scholar] [CrossRef] [PubMed]
- Kern, F. Normal Plasma Cholesterol in an 88-Year-Old Man Who Eats 25 Eggs a Day. N. Engl. J. Med. 1991, 324, 896–899. [Google Scholar] [CrossRef]
- Kuang, H.; Yang, F.; Zhang, Y.; Wang, T.; Chen, G. The Impact of Egg Nutrient Composition and Its Consumption on Cholesterol Homeostasis. Cholesterol 2018, 2018, 6303810. [Google Scholar] [CrossRef]
- Kang, J.W.; Zivkovic, A.M. Are Eggs Good Again? A Precision Nutrition Perspective on the Effects of Eggs on Cardiovascular Risk, Taking into Account Plasma Lipid Profiles and TMAO. J. Nutr. Biochem. 2022, 100, 108906. [Google Scholar] [CrossRef]
- Lütjohann, D.; Meese, C.O.; Crouse, J.R., 3rd; von Bergmann, K. Evaluation of deuterated cholesterol and deuterated sitostanol for measurement of cholesterol absorption in humans. J. Lipid Res. 1993, 34, 1039–1046. [Google Scholar] [CrossRef]
- Bosner, M.S.; Ostlund, R.E., Jr.; Osofisan, O.; Grosklos, J.; Fritschle, C.; Lange, L.G. Assessment of percent cholesterol absorption in humans with stable isotopes. J. Lipid Res. 1993, 34, 1047–1053. [Google Scholar] [CrossRef]
- Turley, S.D.; Spady, D.K.; Dietschy, J.M. Role of liver in the synthesis of cholesterol and the clearance of low density lipoproteins in the cynomolgus monkey. J. Lipid Res. 1995, 36, 67–79. [Google Scholar] [CrossRef]
- Van Erpecum, K.J.; van Berge Henegouwen, G.P.; Stolk, M.F.; Hopman, W.P.; Jansen, J.B.; Lamers, C.B. Fasting gallbladder volume, postprandial emptying and cholecystokinin release in gallstone patients and normal subjects. J. Hepatol. 1992, 14, 194–202. [Google Scholar] [CrossRef]
- Juste, C.; Gérard, P. Cholesterol-to-Coprostanol Conversion by the Gut Microbiota: What We Know, Suspect, and Ignore. Microorganisms 2021, 9, 1881. [Google Scholar] [CrossRef] [PubMed]
- Villette, R.; Kc, P.; Beliard, S.; Tapia, M.F.S.; Rainteau, D.; Guerin, M.; Lesnik, P. Unraveling Host-Gut Microbiota Dialogue and Its Impact on Cholesterol Levels. Front. Pharmacol. 2020, 11, 278. [Google Scholar] [CrossRef]
- Kenny, D.J.; Plichta, D.R.; Shungin, D.; Koppel, N.; Hall, A.B.; Fu, B.; Vasan, R.S.; Shaw, S.Y.; Vlamakis, H.; Balskus, E.P.; et al. Cholesterol Metabolism by Uncultured Human Gut Bacteria Influences Host Cholesterol Level. Cell Host Microbe 2020, 28, 245–257. [Google Scholar] [CrossRef]
- Vors, C.; Joumard-Cubizolles, L.; Lecomte, M.; Combe, E.; Ouchchane, L.; Dra, J.; Raynal, K.; Joffre, F.; Meiller, L.; Le Barz, M.; et al. Milk polar lipids reduce lipid cardiovascular risk factors in overweight postmenopausal women: Towards a gut sphingomyelin-cholesterol interplay. Gut 2020, 69, 487–501. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, A.; Koutkia, P.; Apovian, C. Nutritional Management of Short Bowel Syndrome in Adults. J. Clin. Gastroenterol. 2002, 34, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Glaysher, M.A.; Ward, J.; Aldhwayan, M.; Ruban, A.; Prechtl, C.G.; Fisk, H.L.; Chhina, N.; Al-Najim, W.; Smith, C.; Klimow-ska-Nassar, N.; et al. The effect of a duo-denal-jejunal bypass liner on lipid profile and blood concentrations of long chain polyunsaturated fatty acids. Clin. Nutr. 2021, 40, 2343–2354. [Google Scholar] [CrossRef]
- Altmann, S.W.; Davis, H.R., Jr.; Zhu, L.J.; Yao, X.; Hoos, L.M.; Tetzloff, G.; Iyer, S.P.; Maguire, M.; Golovko, A.; Zeng, M.; et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 2004, 303, 1201–1204. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, J.; Hussain, M.M. Evidence for multiple complementary pathways for efficient cholesterol absorption in mice. J. Lipid Res. 2005, 46, 1491–1501. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.M. Intestinal lipid absorption and lipoprotein formation. Curr. Opin. Lipidol. 2014, 25, 200–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weggemans, R.M.; Zock, P.L.; Tai, E.S.; Ordovas, J.M.; Molhuizen, H.O.; Katan, M.B. ATP binding cassette G5 C1950G poly-morphism may affect blood cholesterol concentrations in humans. Clin. Genet. 2002, 62, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Graf, G.A.; Yu, L.; Li, W.P.; Gerard, R.; Tuma, P.L.; Cohen, J.C.; Hobbs, H.H. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J. Biol. Chem. 2003, 278, 48275–48282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalivianakis, M.; Minich, D.M.; Bijleveld, C.M.; van Aalderen, W.M.; Stellaard, F.; Laseur, M.; Vonk, R.J.; Verkade, H.J. Fat malabsorption in cystic fibrosis patients receiving enzyme replacement therapy is due to impaired intestinal uptake of long-chain fatty acids. Am. J. Clin. Nutr. 1999, 69, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Kalivianakis, M.; Elstrodt, J.; Havinga, R.; Kuipers, F.; Stellaard, F.; Sauer, P.J.; Vonk, R.J.; Verkade, H.J. Validation in an animal model of the carbon 13-labeled mixed triglyceride breath test for the detection of intestinal fat malabsorption. J. Pediatr. 1999, 135, 444–450. [Google Scholar] [CrossRef]
- Lütjohann, D.; von Bergmann, K. Phytosterolaemia: Diagnosis, characterization and therapeutical approaches. Ann. Med. 1997, 29, 181–184. [Google Scholar] [CrossRef]
- Berge, K.E.; von Bergmann, K.; Lutjohann, D.; Guerra, R.; Grundy, S.M.; Hobbs, H.H.; Cohen, J.C. Heritability of plasma noncholesterol sterols and relationship to DNA sequence polymorphism in ABCG5 and ABCG8. J. Lipid Res. 2002, 43, 486–494. [Google Scholar] [CrossRef]
- Lütjohann, D.; Björkhem, I.; Beil, U.F.; von Bergmann, K. Sterol absorption and sterol balance in phytosterolemia evaluated by deuterium-labeled sterols: Effect of sitostanol treatment. J. Lipid Res. 1995, 36, 1763–1773. [Google Scholar] [CrossRef]
- Zanlungo, S.; Nervi, F. Discovery of the hepatic canalicular and intestinal cholesterol transporters. New targets for treatment of hypercholesterolemia. Eur. Rev. Med. Pharmacol. Sci. 2003, 7, 33–39. [Google Scholar]
- Yu, L.; Gupta, S.; Xu, F.; Liverman, A.D.; Moschetta, A.; Mangelsdorf, D.J.; Repa, J.J.; Hobbs, H.H.; Cohen, J.C. Expression of ABCG5 and ABCG8 is required for regulation of biliary cholesterol secretion. J. Biol. Chem. 2005, 280, 8742–8747. [Google Scholar] [CrossRef] [Green Version]
- Gylling, H.; Miettinen, T.A. Serum cholesterol and cholesterol and lipoprotein metabolism in hypercholesterolaemic NIDDM patients before and during sitostanol ester-margarine treatment. Diabetologia 1994, 37, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, T.A.; Gylling, H. Regulation of cholesterol metabolism by dietary plant sterols. Curr. Opin. Lipidol. 1999, 10, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.C. The beneficial effects of plant sterols on serum cholesterol. Can. J. Cardiol. 2001, 17, 715–721. [Google Scholar] [PubMed]
- Mansbach, C.M.; Siddiqi, S.A. The biogenesis of chylomicrons. Annu. Rev. Physiol. 2010, 72, 315–333. [Google Scholar] [CrossRef] [Green Version]
- Auclair, N.; Melbouci, L.; St-Pierre, D.; Levy, E. Gastrointestinal factors regulating lipid droplet formation in the intestine. Exp. Cell Res. 2018, 363, 1–14. [Google Scholar] [CrossRef]
- Jakulj, L.; van Dijk, T.H.; de Boer, J.F.; Kootte, R.S.; Schonewille, M.; Paalvast, Y.; Boer, T.; Bloks, V.W.; Boverhof, R.; Nieuwdorp, M.; et al. Transintestinal cholesterol transport is active in mice and humans and controls ezetimibe-induced fecal neutral sterol excretion. Cell Metab. 2016, 24, 783–794. [Google Scholar] [CrossRef] [Green Version]
- Russell, D.W. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 2003, 72, 137–174. [Google Scholar] [CrossRef] [Green Version]
- Sudhop, T.; Reber, M.; Tribble, D.; Sapre, A.; Taggart, W.; Gibbons, P.; Musliner, T.; von Bergmann, K.; Lütjohann, D. Changes in cholesterol absorption and cholesterol synthesis caused by ezetimibe and/or simvastatin in men. J. Lipid Res. 2009, 50, 2117–2123. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.J.; Schoeller, D.A. Evidence for diurnal periodicity in human cholesterol synthesis. J. Lipid Res. 1990, 31, 667–673. [Google Scholar] [CrossRef]
- Parker, T.S.; McNamara, D.J.; Brown, C.; Garrigan, O.; Kolb, R.; Batwin, H.; Ahrens, E.H., Jr. Mevalonic acid in human plasma: Relationship of concentration and circadian rhythm to cholesterol synthesis rates in man. Proc. Natl. Acad. Sci. USA 1982, 79, 3037–3041. [Google Scholar] [CrossRef] [Green Version]
- Gälman, C.; Angelin, B.; Rudling, M. Bile acid synthesis in humans has a rapid diurnal variation that is asynchronous with cholesterol synthesis. Gastroenterology 2005, 129, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Honda, A.; Tanaka, N.; Matsuzaki, Y.; Shoda, J.; He, B.; Osuga, T.; Miyazaki, H. Determination of 7 al-pha-hydroxy-4-cholesten-3-one level in plasma using isotope-dilution mass spectrometry and monitoring its circadian rhythm in human as an index of bile acid biosynthesis. J. Chromatogr. B Biomed. Appl. 1994, 655, 179–187. [Google Scholar] [CrossRef]
- Stellaard, F.; Lütjohann, D. Dynamics of the enterohepatic circulation of bile acids in healthy humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G55–G66. [Google Scholar] [CrossRef] [PubMed]
- van der Velde, A.E.; Vrins, C.L.; van den Oever, K.; Seemann, I.; Oude Elferink, R.P.; van Eck, M.; Kuipers, F.; Groen, A.K. Regulation of direct transintestinal cholesterol excretion in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G203–G208. [Google Scholar] [CrossRef] [Green Version]
- Reeskamp, L.F.; Meessen, E.C.E.; Groen, A.K. Transintestinal cholesterol excretion in humans. Curr. Opin. Lipidol. 2018, 29, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Van de Peppel, I.P.; Bertolini, A.; van Dijk, T.H.; Groen, A.K.; Jonker, J.W.; Verkade, H.J. Efficient reabsorption of transintestinally excreted cholesterol is a strong determinant for cholesterol disposal in mice. J. Lipid Res. 2019, 60, 1562–1572. [Google Scholar] [CrossRef] [Green Version]
- Heidemann, B.E.; Koopal, C.; Bots, M.L.; Asselbergs, F.W.; Westerink, J.; Visseren, F.L. The relation between VLDL-cholesterol and risk of cardiovascular events in patients with manifest cardiovascular disease. J. Int. J. Cardiol. 2021, 322, 251–257. [Google Scholar] [CrossRef]
- Balling, M.; Afzal, S.; Varbo, A.; Langsted, A.; Davey Smith, G.; Nordestgaard, B.G. VLDL Cholesterol Accounts for One-Half of the Risk of Myocardial Infarction Associated With apoB-Containing Lipoproteins. J. Am. Coll. Cardiol. 2020, 76, 2725–2735. [Google Scholar] [CrossRef]
- Sniderman, A.; McQueen, M.; Contois, J.; Williams, K.; Furberg, C.D. Why is non-high-density lipoprotein cholesterol a better marker of the risk of vascular disease than low-density lipoprotein cholesterol? J. Clin. Lipidol. 2010, 4, 152–155. [Google Scholar] [CrossRef]
- Brown, W.V.; Myers, G.L.; Sniderman, A.D.; Stein, E. Should we use apoB for risk assessment and as a target for treatment? J. Clin. Lipidol. 2010, 4, 144–151. [Google Scholar] [CrossRef]
- Sniderman, A.D.; Navar, A.M.; Thanassoulis, G. Apolipoprotein B vs. Low-Density Lipoprotein Cholesterol and Non-High-Density Lipoprotein Cholesterol as the Primary Measure of Apolipoprotein B Lipoprotein-Related Risk: The Debate Is Over. JAMA Cardiol. 2022, 7, 257–258. [Google Scholar] [CrossRef] [PubMed]
- Ip, S.; Lichtenstein, A.H.; Chung, M.; Lau, J.; Balk, E.M. Systematic review: Association of low-density lipoprotein subfractions with cardiovascular outcomes. Ann. Intern. Med. 2009, 150, 474–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajman, I.; Kendall, M.J.; Cramb, R.; Holder, R.L.; Salih, M.; Gammage, M.D. Investigation of low density lipoprotein subfractions as a coronary risk factor in normotriglyceridaemic men. Atherosclerosis 1996, 125, 231–242. [Google Scholar] [CrossRef]
- Froyen, E. The effects of fat consumption on low-density lipoprotein particle size in healthy individuals: A narrative review. Lipids Health Dis. 2021, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.C.F.; Raposo, H.F. Cholesteryl Ester Transfer Protein and Lipid Metabolism and Cardiovascular Diseases. Adv. Exp. Med. Biol. 2020, 1276, 15–25. [Google Scholar] [PubMed]
- Taheri, H.; Filion, K.B.; Windle, S.B.; Reynier, P.; Eisenberg, M.J. Cholesteryl Ester Transfer Protein Inhibitors and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cardiology 2020, 145, 236–250. [Google Scholar] [CrossRef]
- Banerjee, S.; De, A. Pathophysiology and inhibition of cholesteryl ester transfer protein for prevention of cardiovascular diseases: An update. Drug Discov. Today. 2021, 26, 1759–1764. [Google Scholar] [CrossRef]
- Grundy, S.M.; Ahrens, E.H., Jr. Measurements of cholesterol turnover, synthesis, and absorption in man, carried out by isotope kinetic and sterol balance methods. J. Lipid Res. 1969, 10, 91–107. [Google Scholar] [CrossRef]
- Hellerstein, M.K.; Neese, R.A. Mass isotopomer distribution analysis: A technique for measuring biosynthesis and turnover of polymers. Am. J. Physiol. 1992, 263, E988–E1001. [Google Scholar] [CrossRef]
- Jones, P.J.; Leitch, C.A.; Li, Z.C.; Connor, W.E. Human cholesterol synthesis measurement using deuterated water. Theoretical and procedural considerations. Arterioscler. Thromb. 1993, 13, 247–253. [Google Scholar] [CrossRef] [Green Version]
- Kempen, H.J.; Glatz, J.F.; Gevers Leuven, J.A.; van der Voort, H.A.; Katan, M.B. Serum lathosterol concentration is an indicator of whole-body cholesterol synthesis in humans. J. Lipid Res. 1988, 29, 1149–1155. [Google Scholar] [CrossRef]
- Tilvis, R.S.; Miettinen, T.A. Serum plant sterols and their relation to cholesterol absorption. Am. J. Clin. Nutr. 1986, 43, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, T.; Axtmann, G.; von Bergmann, K. Comparison of intestinal absorption of cholesterol with different plant sterols in man. Eur. J. Clin. Invest. 1993, 23, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Stellaard, F.; Sackmann, M.; Sauerbruch, T.; Paumgartner, G. Simultaneous determination of cholic acid and chenodeoxycholic acid pool sizes and fractional turnover rates in human serum using 13C-labeled bile acids. J. Lipid Res. 1984, 25, 1313–1319. [Google Scholar] [CrossRef]
- Hulzebos, C.V.; Renfurm, L.; Bandsma, R.H.; Verkade, H.J.; Boer, T.; Boverhof, R.; Tanaka, H.; Mierau, I.; Sauer, P.J.; Kuipers, F.; et al. Measurement of parameters of cholic acid kinetics in plasma using a microscale stable isotope dilution technique: Application to rodents and humans. J. Lipid Res. 2001, 42, 1923–1929. [Google Scholar] [CrossRef]
- Hahn, C.; Reichel, C.; von Bergmann, K. Serum concentration of 7 alpha-hydroxycholesterol as an indicator of bile acid synthesis in humans. J. Lipid Res. 1995, 36, 2059–2066. [Google Scholar] [CrossRef]
- Axelson, M.; Aly, A.; Sjövall, J. Levels of 7 alpha-hydroxy-4-cholesten-3-one in plasma reflect rates of bile acid synthesis in man. FEBS Lett. 1988, 239, 324–328. [Google Scholar] [CrossRef] [Green Version]
- Magkos, F.; Sidossis, L.S. Measuring very low density lipoprotein-triglyceride kinetics in man in vivo: How different the various methods really are. Curr. Opin. Clin. Nutr. Metab. Care. 2004, 7, 547–555. [Google Scholar] [CrossRef]
- Chan, D.C.; Watts, G.F. Recent advances in the investigation of lipoprotein metabolism using tracer methodology. Clin. Lab. 2006, 52, 353–361. [Google Scholar]
- Lütjohann, D.; Stellaard, F.; Mulder, M.T.; Sijbrands, E.J.G.; Weingärtner, O. The emerging concept of “individualized choles-terol-lowering therapy𠇍: A change in paradigm. Pharmacol. Ther. 2019, 199, 111–116. [Google Scholar] [CrossRef]
- Luo, J.; Yang, H.; Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, X.H.; Ou, X.; Ouyang, X.P.; Tang, C.K. Hepatic cholesterol transport and its role in non-alcoholic fatty liver disease and atherosclerosis. Prog. Lipid Res. 2021, 83, 101109. [Google Scholar] [CrossRef] [PubMed]
- Weingärtner, O.; Baber, R.; Teupser, D. Plant sterols in food: No consensus in guidelines. Biochem. Biophys. Res. Commun. 2014, 446, 811–813. [Google Scholar] [CrossRef] [PubMed]
- Weingärtner, O.; Teupser, D.; Patel, S.B. The Atherogenicity of Plant Sterols: The Evidence from Genetics to Clinical Trials. J. AOAC Int. 2015, 98, 742–749. [Google Scholar] [CrossRef]
- Pirillo, A.; Catapano, A.L. New insights into the role of bempedoic acid and ezetimibe in the treatment of hypercholesterolemia. Curr. Opin. Endocrinol. Diabetes Obes. 2022, 29, 161–166. [Google Scholar] [CrossRef]
- Bagepally, B.S.; Sasidharan, A. Incremental net benefit of lipid-lowering therapy with PCSK9 inhibitors: A systematic review and meta-analysis of cost-utility studies. Eur. J. Clin. Pharmacol. 2022, 78, 351–363. [Google Scholar] [CrossRef]
- Smith, K.W.; White, C.M. Inclisiran: A Novel Small Interfering RNA Drug for Low-Density Lipoprotein Reduction. J. Clin. Pharmacol. 2022. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stellaard, F. From Dietary Cholesterol to Blood Cholesterol, Physiological Lipid Fluxes, and Cholesterol Homeostasis. Nutrients 2022, 14, 1643. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14081643
Stellaard F. From Dietary Cholesterol to Blood Cholesterol, Physiological Lipid Fluxes, and Cholesterol Homeostasis. Nutrients. 2022; 14(8):1643. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14081643
Chicago/Turabian StyleStellaard, Frans. 2022. "From Dietary Cholesterol to Blood Cholesterol, Physiological Lipid Fluxes, and Cholesterol Homeostasis" Nutrients 14, no. 8: 1643. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14081643