The Mediating Effect of Inflammation between the Dietary and Health-Related Behaviors and Metabolic Syndrome in Adolescence
Abstract
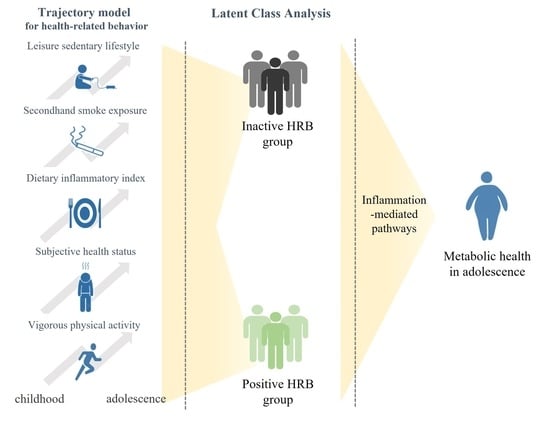
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Exposure
2.3. Mediator
2.4. Outcome
2.5. Covariates
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranasinghe, P.; Mathangasinghe, Y.; Jayawardena, R.; Hills, A.P.; Misra, A. Prevalence and trends of metabolic syndrome among adults in the asia-pacific region: A systematic review. BMC Public Health 2017, 17, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belete, R.; Ataro, Z.; Abdu, A.; Sheleme, M. Global prevalence of metabolic syndrome among patients with type I diabetes mellitus: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2021, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Friend, A.; Craig, L.; Turner, S. The prevalence of metabolic syndrome in children: A systematic review of the literature. Metab. Syndr. Relat. Disord. 2013, 11, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.H.; Kang, D.R.; Kim, J.Y.; Koh, K.K. Metabolic syndrome fact sheet 2021: Executive report. CardioMetabolic Syndr. J. 2021, 1, 125–134. [Google Scholar] [CrossRef]
- Baird, J.; Jacob, C.; Barker, M.; Fall, C.H.; Hanson, M.; Harvey, N.C.; Inskip, H.M.; Kumaran, K.; Cooper, C. Developmental Origins of Health and Disease: A Lifecourse Approach to the Prevention of Non-Communicable Diseases. Healthcare 2017, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Al-Hamad, D.; Raman, V. Metabolic syndrome in children and adolescents. Transl. Pediatr. 2017, 6, 397–407. [Google Scholar] [CrossRef] [Green Version]
- Brage, S.; Wedderkopp, N.; Ekelund, U.; Franks, P.W.; Wareham, N.J.; Andersen, L.B.; Froberg, K. Features of the metabolic syndrome are associated with objectively measured physical activity and fitness in Danish children: The European Youth Heart Study (EYHS). Diabetes Care 2004, 27, 2141–2148. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Schoufour, J.; Wang, D.D.; Dhana, K.; Pan, A.; Liu, X.; Song, M.; Liu, G.; Shin, H.J.; Sun, Q.; et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: Prospective cohort study. bmj 2020, 368, l6669. [Google Scholar] [CrossRef] [Green Version]
- Yudkin, J.S.; Kumari, M.; Humphries, S.E.; Mohamed-Ali, V. Inflammation, obesity, stress and coronary heart disease: Is interleukin-6 the link? Atherosclerosis 2000, 148, 209–214. [Google Scholar] [CrossRef]
- Miranda, V.P.N.; Coimbra, D.R.; Bastos, R.R.; Miranda Júnior, M.V.; Amorim, P. Use of latent class analysis as a method of assessing the physical activity level, sedentary behavior and nutritional habit in the adolescents’ lifestyle: A scoping review. PLoS ONE 2021, 16, e0256069. [Google Scholar] [CrossRef]
- Grøntved, A.; Ried-Larsen, M.; Møller, N.C.; Kristensen, P.L.; Wedderkopp, N.; Froberg, K.; Hu, F.B.; Ekelund, U.; Andersen, L.B. Youth screen-time behaviour is associated with cardiovascular risk in young adulthood: The European Youth Heart Study. Eur. J. Prev. Cardiol. 2014, 21, 49–56. [Google Scholar] [CrossRef]
- Mozzillo, E.; Zito, E.; Maffeis, C.; De Nitto, E.; Maltoni, G.; Marigliano, M.; Zucchini, S.; Franzese, A.; Valerio, G. Unhealthy lifestyle habits and diabetes-specific health-related quality of life in youths with type 1 diabetes. Acta Diabetol. 2017, 54, 1073–1080. [Google Scholar] [CrossRef]
- Morrison, J.A.; Friedman, L.A.; Wang, P.; Glueck, C.J. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J. Pediatr. 2008, 152, 201–206. [Google Scholar] [CrossRef]
- Lee, H.A.; Park, B.; Min, J.; Choi, E.J.; Kim, U.J.; Park, H.J.; Park, E.A.; Cho, S.J.; Kim, H.S.; Lee, H.; et al. Cohort profile: The Ewha Birth and Growth Study. Epidemiol. Health 2021, 43, e2021016. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [Green Version]
- Eisenmann, J.C. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovasc. Diabetol. 2008, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Shafiee, G.; Kelishadi, R.; Heshmat, R.; Qorbani, M.; Motlagh, M.E.; Aminaee, T.; Ardalan, G.; Taslimi, M.; Poursafa, P.; Larijani, B. First report on the validity of a continuous Metabolic syndrome score as an indicator for Metabolic syndrome in a national sample of paediatric population—The CASPIAN-III study. Endokrynol. Pol. 2013, 64, 278–284. [Google Scholar] [CrossRef] [Green Version]
- Heshmat, R.; Heidari, M.; Ejtahed, H.S.; Motlagh, M.E.; Mahdavi-Gorab, A.; Ziaodini, H.; Taheri, M.; Shafiee, G.; Beshtar, S.; Qorbani, M.; et al. Validity of a continuous metabolic syndrome score as an index for modeling metabolic syndrome in children and adolescents: The CASPIAN-V study. Diabetol. Metab. Syndr. 2017, 9, 89. [Google Scholar] [CrossRef] [Green Version]
- Meader, N.; King, K.; Moe-Byrne, T.; Wright, K.; Graham, H.; Petticrew, M.; Power, C.; White, M.; Sowden, A.J. A systematic review on the clustering and co-occurrence of multiple risk behaviours. BMC Public Health 2016, 16, 657. [Google Scholar] [CrossRef] [Green Version]
- McAloney, K.; Graham, H.; Law, C.; Platt, L. A scoping review of statistical approaches to the analysis of multiple health-related behaviours. Prev. Med. 2013, 56, 365–371. [Google Scholar] [CrossRef]
- Jones, B.L.; Nagin, D.S. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol. Methods Res. 2007, 35, 542–571. [Google Scholar] [CrossRef] [Green Version]
- Hagenaars, J.A.; McCutcheon, A.L. Applied Latent Class Analysis; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Tein, J.Y.; Coxe, S.; Cham, H. Statistical Power to Detect the Correct Number of Classes in Latent Profile Analysis. Struct. Equ. Modeling Multidiscip. J. 2013, 20, 640–657. [Google Scholar] [CrossRef]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach; Guilford Publications: New York, NY, USA, 2017. [Google Scholar]
- Yim, E.; Lee, K.; Park, I.; Lee, S. The Prevalence of Metabolic Syndrome and Health-Related Behavior Changes: The Korea National Health Examination Survey. Healthcare 2020, 8, 134. [Google Scholar] [CrossRef]
- Santos, A.C.; Ebrahim, S.; Barros, H. Alcohol intake, smoking, sleeping hours, physical activity and the metabolic syndrome. Prev. Med. 2007, 44, 328–334. [Google Scholar] [CrossRef]
- Wennberg, P.; Gustafsson, P.E.; Dunstan, D.W.; Wennberg, M.; Hammarström, A. Television viewing and low leisure-time physical activity in adolescence independently predict the metabolic syndrome in mid-adulthood. Diabetes Care 2013, 36, 2090–2097. [Google Scholar] [CrossRef] [Green Version]
- Ambrosini, G.L.; Huang, R.C.; Mori, T.A.; Hands, B.P.; O’Sullivan, T.A.; de Klerk, N.H.; Beilin, L.J.; Oddy, W.H. Dietary patterns and markers for the metabolic syndrome in Australian adolescents. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 274–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.P.; Chen, G.C.; Wang, X.P.; Qin, L.; Bai, Y. Dietary Fiber and Metabolic Syndrome: A Meta-Analysis and Review of Related Mechanisms. Nutrients 2017, 10, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, B.; Liu, Y.; Lin, X.; Fang, Y.; Cui, J.; Wan, J. Dietary fiber intake and risk of metabolic syndrome: A meta-analysis of observational studies. Clin. Nutr. 2018, 37, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Abdollahzad, H.; Pasdar, Y.; Nachvak, S.M.; Rezaeian, S.; Saber, A.; Nazari, R. The Relationship Between the Dietary Inflammatory Index and Metabolic Syndrome in Ravansar Cohort Study. Diabetes Metab. Syndr. Obes. 2020, 13, 477–487. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Dan, H.; Pang, Y.; Kim, B.; Jeong, H.; Lee, J.E.; Kim, O. Association between Dietary Habits, Shift Work, and the Metabolic Syndrome: The Korea Nurses’ Health Study. Int. J. Environ. Res. Public Health 2020, 17, 7697. [Google Scholar] [CrossRef]
- Raposa, E.B.; Bower, J.E.; Hammen, C.L.; Najman, J.M.; Brennan, P.A. A developmental pathway from early life stress to inflammation: The role of negative health behaviors. Psychol. Sci. 2014, 25, 1268–1274. [Google Scholar] [CrossRef] [Green Version]
- Yudkin, J.S.; Juhan-Vague, I.; Hawe, E.; Humphries, S.E.; di Minno, G.; Margaglione, M.; Tremoli, E.; Kooistra, T.; Morange, P.E.; Lundman, P.; et al. Low-grade inflammation may play a role in the etiology of the metabolic syndrome in patients with coronary heart disease: The HIFMECH study. Metabolism 2004, 53, 852–857. [Google Scholar] [CrossRef]
- Berlin, K.S.; Williams, N.A.; Parra, G.R. An introduction to latent variable mixture modeling (part 1): Overview and cross-sectional latent class and latent profile analyses. J. Pediatr. Psychol. 2014, 39, 174–187. [Google Scholar] [CrossRef] [Green Version]
- Miettunen, J.; Nordström, T.; Kaakinen, M.; Ahmed, A.O. Latent variable mixture modeling in psychiatric research--a review and application. Psychol. Med. 2016, 46, 457–467. [Google Scholar] [CrossRef]
- The 17th (2021) Korea Youth Risk Behavior Web-Based Survey Statistics Report; Ministry of Education, Ministry of Health & Welfare, Korea Disease Control and Prevention Agency: Cheongju, Korea, 2021.



| Total (n = 249) | Boys (n = 122) | Girls (n = 127) | p-Value | |
|---|---|---|---|---|
| Age (years) | 13.28 ± 0.59 | 13.24 ± 0.53 | 13.31 ± 0.64 | 0.301 |
| Subjective health status * | 183 (58.9%) | 96 (39.0%) | 87 (35.4%) | 0.223 |
| Vigorous physical activity (more than 20 min) | ||||
| Never | 58 (23.3%) | 19 (15.6%) | 39 (31.2%) | 0.004 |
| 1–2 times/week | 95 (38.5%) | 45 (36.9%) | 50 (40.0%) | |
| 3–4 times/week | 73 (29.6%) | 43 (35.3%) | 30 (24.0%) | |
| ≥5 times/week | 21 (8.5%) | 15 (12.3%) | 6 (4.8%) | |
| Sedentary lifestyle | ||||
| Never | 4 (1.63%) | 1 (0.8%) | 3 (2.4%) | 0.988 |
| Less than 1 h/day | 64 (26.0%) | 31 (26.4%) | 33 (26.4%) | |
| 1–2 h/day | 73 (29.7%) | 36 (29.8%) | 37 (29.6%) | |
| More than 2 h/day | 105 (42.7%) | 53 (43.8%) | 52 (41.6%) | |
| Dietary Inflammation Index | 0.00 ± 1.85 | −0.25 ± 1.72 | 0.24 ± 1.95 | 0.037 |
| hs-CRP (mg/dL) | 0.16 (0.11, 0.38) | 0.20 (0.11, 0.48) | 0.11 (0.11, 0.33) | 0.013 |
| IL-6 (pg/mL) | 2.74 (2.02, 3.65) | 2.72 (2.01, 3.48) | 2.76 (2.08, 3.76) | 0.788 |
| cMetS | 0.00 ± 3.03 | 0.00 ± 3.15 | 0.00 ± 2.92 | 0.425 |
| Monthly household income, KRW | ||||
| <KRW 3 million | 16 (6.6%) | 7 (5.8%) | 9 (7.3%) | 0.739 |
| KRW 3–5 million | 68 (27.9%) | 34 (28.1%) | 34 (27.6%) | |
| ≥KRW 5 million | 160 (65.6%) | 80 (66.1%) | 80 (65.0%) | |
| hs-CRP | IL-6 | cMetS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | p-Value | β | SE | p-Value | β | SE | p-Value | ||
| Crude model | Inactive HRB group | 0.228 | 0.124 | 0.067 | 0.187 | 0.069 | 0.007 | 0.921 | 0.392 | 0.02 |
| Positive HRB Group | ||||||||||
| Adjusted model | Inactive HRB group | 0.202 | 0.129 | 0.118 | 0.168 | 0.072 | 0.02 | 0.751 | 0.405 | 0.065 |
| Positive HRB group | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, U.-J.; Choi, E.-J.; Park, H.; Lee, H.-A.; Park, B.; Kim, H.; Hong, Y.; Jung, S.; Park, H. The Mediating Effect of Inflammation between the Dietary and Health-Related Behaviors and Metabolic Syndrome in Adolescence. Nutrients 2022, 14, 2339. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14112339
Kim U-J, Choi E-J, Park H, Lee H-A, Park B, Kim H, Hong Y, Jung S, Park H. The Mediating Effect of Inflammation between the Dietary and Health-Related Behaviors and Metabolic Syndrome in Adolescence. Nutrients. 2022; 14(11):2339. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14112339
Chicago/Turabian StyleKim, Ui-Jeong, Eun-Jeong Choi, Hyunjin Park, Hye-Ah Lee, Bomi Park, Haesoon Kim, Youngsun Hong, Seungyoun Jung, and Hyesook Park. 2022. "The Mediating Effect of Inflammation between the Dietary and Health-Related Behaviors and Metabolic Syndrome in Adolescence" Nutrients 14, no. 11: 2339. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14112339