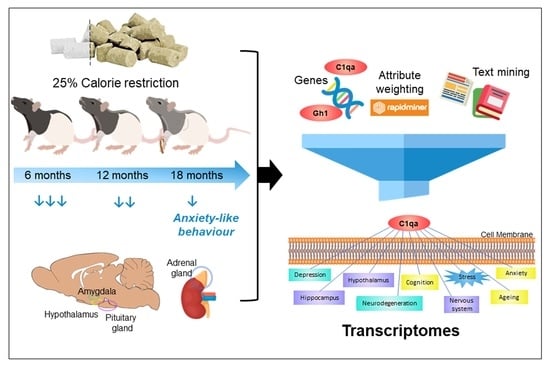
Long-Term Calorie Restriction Alters Anxiety-like Behaviour and the Brain and Adrenal Gland Transcriptomes of the Ageing Male Rat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Dietary Treatment
2.3. Behavioural Testing
2.3.1. Elevated Plus Maze
2.3.2. Open Field Test
2.3.3. Acoustic Startle
2.4. RNA Preparation and Sequencing
2.5. Data and Statistical Analysis of Body Weight and Behavioural Data
2.6. Transcriptomic Data Analysis
2.7. Signature Discovery by Attribute Weighting (Feature Selection) Models
2.8. Text Mining and Integrative Network Analysis of Expressed Genes with Behaviour
3. Results
3.1. Body Weight
3.2. Elevated Plus Maze
3.3. Open Field
3.4. Acoustic Startle
3.5. Tissue-Based Response to Long-Term CR: Genes with Significant Differential Expression in Each Region of Interest
3.6. Tissue-Independent Transcriptomic Signature of Long-Term CR: Application of Attribute Weighting Analysis of Gene Expression
3.7. Text Mining-Based Network Analysis Links Long-Term CR Transcriptomic Signature with Behaviour (Anxiety, Depression, Cognition), Ageing and HPA-Axis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bryant, C.; Jackson, H.; Ames, D. Depression and anxiety in medically unwell older adults: Prevalence and short-term course. Int. Psychogeriatr. 2009, 21, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Wolitzky-Taylor, K.B.; Castriotta, N.; Lenze, E.J.; Stanley, M.A.; Craske, M.G. Anxiety disorders in older adults: A comprehensive review. Depress. Anxiety 2010, 27, 190–211. [Google Scholar] [CrossRef] [PubMed]
- Balsamo, M.; Cataldi, F.; Carlucci, L.; Fairfield, B. Assessment of anxiety in older adults: A review of self-report measures. Clin. Interv. Aging 2018, 13, 573–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreescu, C.; Lee, S. Anxiety disorders in the elderly. Adv. Exp. Med. Biol. 2020, 1191, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Energy intake, meal frequency, and health: A neurobiological perspective. Annu. Rev. Nutr. 2005, 25, 237–260. [Google Scholar] [CrossRef] [Green Version]
- Masoro, E.J. Overview of caloric restriction and ageing. Mech. Ageing Dev. 2005, 126, 913–922. [Google Scholar] [CrossRef]
- Omodei, D.; Fontana, L. Calorie restriction and prevention of age-associated chronic disease. FEBS Lett. 2011, 585, 1537–1542. [Google Scholar] [CrossRef] [Green Version]
- Redman, L.M.; Smith, S.R.; Burton, J.H.; Martin, C.K.; Il’yasova, D.; Ravussin, E. Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metab. 2018, 27, 805–815.e4. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Zou, L.; Kong, Z.; Yang, L. Cognitive impact of calorie restriction: A narrative review. J. Am. Med. Dir. Assoc. 2020, 21, 1394–1401. [Google Scholar] [CrossRef]
- Genn, R.F.; Tucci, S.A.; Thomas, A.; Edwards, J.E.; File, S.E. Age-associated sex differences in response to food deprivation in two animal tests of anxiety. Neurosci. Biobehav. Rev. 2003, 27, 155–161. [Google Scholar] [CrossRef]
- Guccione, L.; Paolini, A.G.; Penman, J.; Djouma, E. The effects of calorie restriction on operant-responding for alcohol in the alcohol preferring (iP) rat. Behav. Brain Res. 2012, 230, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Guccione, L.; Djouma, E.; Penman, J.; Paolini, A.G. Calorie restriction inhibits relapse behaviour and preference for alcohol within a two-bottle free choice paradigm in the alcohol preferring (iP) rat. Physiol. Behav. 2013, 110–111, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Kenny, R.; Dinan, T.; Cai, G.; Spencer, S.J. Effects of mild calorie restriction on anxiety and hypothalamic-pituitary-adrenal axis responses to stress in the male rat. Physiol. Rep. 2014, 2, e00265. [Google Scholar] [CrossRef] [PubMed]
- Levay, E.A.; Govic, A.; Penman, J.; Paolini, A.G.; Kent, S. Effects of adult-onset calorie restriction on anxiety-like behavior in rats. Physiol. Behav. 2007, 92, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Tanahashi, T.; Kawai, T.; Chikahisa, S.; Katsuura, S.; Nishida, K.; Teshima-Kondo, S.; Sei, H.; Rokutan, K. Changes in behavior and gene expression induced by caloric restriction in C57BL/6 mice. Physiol. Genom. 2009, 39, 227–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maniscalco, J.W.; Zheng, H.; Gordon, P.J.; Rinaman, L. Negative energy balance blocks neural and behavioral responses to acute stress by “silencing” central glucagon-like peptide 1 signaling in rats. J. Neurosci. 2015, 35, 10701–10714. [Google Scholar] [CrossRef] [Green Version]
- Toufexis, D.J.; Lipatova, O.; Johnson, A.C.; Abizaid, A. Food-restriction lowers the acoustic startle response in both male and female rats, and, in combination with acute ghrelin injection, abolishes the expression of fear-potentiated startle in male rats. J. Neuroendocr. 2016, 28, 1–7. [Google Scholar] [CrossRef]
- Kuhla, A.; Lange, S.; Holzmann, C.; Maass, F.; Petersen, J.; Vollmar, B.; Wree, A. Lifelong caloric restriction increases working memory in mice. PLoS ONE 2013, 8, e68778. [Google Scholar] [CrossRef] [Green Version]
- Parikh, I.; Guo, J.; Chuang, K.H.; Zhong, Y.; Rempe, R.G.; Hoffman, J.D.; Armstrong, R.; Bauer, B.; Hartz, A.M.; Lin, A.L. Caloric restriction preserves memory and reduces anxiety of aging mice with early enhancement of neurovascular functions. Aging 2016, 8, 2814–2826. [Google Scholar] [CrossRef] [Green Version]
- Prvulovic, M.; Todorovic, S.; Milanovic, D.; Jovic, M.; Vujovic, P.; Kanazir, S.; Mladenovic, A. Calorie restriction changes the anxiety-like behaviour of ageing male Wistar rats in an onset- and duration-dependent manner. Mech. Ageing Dev. 2022, 204, 111666. [Google Scholar] [CrossRef]
- Jimenez, J.C.; Su, K.; Goldberg, A.R.; Luna, V.M.; Biane, J.S.; Ordek, G.; Zhou, P.; Ong, S.K.; Wright, M.A.; Zweifel, L.; et al. Anxiety cells in a hippocampal-hypothalamic circuit. Neuron 2018, 97, 670–683.e6. [Google Scholar] [CrossRef] [Green Version]
- Guarnieri, D.J.; Brayton, C.E.; Richards, S.M.; Maldonado-Aviles, J.; Trinko, J.R.; Nelson, J.; Taylor, J.R.; Gourley, S.L.; DiLeone, R.J. Gene profiling reveals a role for stress hormones in the molecular and behavioral response to food restriction. Biol. Psychiatry 2012, 71, 358–365. [Google Scholar] [CrossRef] [Green Version]
- Saito, K.; Ito, M.; Chiba, T.; Jia, H.; Kato, H. A comparison of gene expression profiles of rat tissues after mild and short-term calorie restrictions. Nutrients 2021, 13, 2277. [Google Scholar] [CrossRef]
- Flak, J.N.; Jankord, R.; Solomon, M.B.; Krause, E.G.; Herman, J.P. Opposing effects of chronic stress and weight restriction on cardiovascular, neuroendocrine and metabolic function. Physiol. Behav. 2011, 104, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Tafet, G.E.; Nemeroff, C.B. Pharmacological treatment of anxiety disorders: The role of the HPA axis. Front. Psychiatry 2020, 11, 443. [Google Scholar] [CrossRef]
- Bürkner, P.C. brms: An R package for Bayesian multilevel models using Stan. J. Stat. Softw. 2017, 80, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Vehtari, A.; Gelman, A.; Simpson, D.; Carpenter, B.; Bürkner, P.-C. Rank-normalization, folding, and localization: An improved Rˆ for assessing convergence of MCMC (with discussion). Bayesian Anal. 2021, 16, 667–718. [Google Scholar] [CrossRef]
- Kruschke, J.K. Bayesian analysis reporting guidelines. Nat. Hum. Behav. 2021, 5, 1282–1291. [Google Scholar] [CrossRef]
- Schwaferts, P.; Augustin, T. Bayesian Decisions Using Regions of Practical Equivalence (ROPE): Foundations; University of Munich: Munich, Germany, 2020. [Google Scholar]
- Makowski, D.; Ben-Shachar, M.; Lüdecke, D. bayestestR: Describing effects and their uncertainty, existence and significance within the bayesian framework. J. Open Source Softw. 2019, 4, 1541. [Google Scholar] [CrossRef]
- Liu, C.H.; Di, Y.P. Analysis of RNA sequencing data using CLC Genomics Workbench. Methods Mol. Biol. 2020, 2102, 61–113. [Google Scholar] [CrossRef]
- Jalili, V.; Afgan, E.; Gu, Q.; Clements, D.; Blankenberg, D.; Goecks, J.; Taylor, J.; Nekrutenko, A. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2020 update. Nucleic Acids Res. 2020, 48, W395–W402. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Mohammadi-Dehcheshmeh, M.; Ebrahimie, E.; Petrovski, K.R. Comprehensive analysis of machine learning models for prediction of sub-clinical mastitis: Deep Learning and Gradient-Boosted Trees outperform other models. Comput. Biol. Med. 2019, 114, 103456. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimie, E.; Ebrahimi, F.; Ebrahimi, M.; Tomlinson, S.; Petrovski, K.R. A large-scale study of indicators of sub-clinical mastitis in dairy cattle by attribute weighting analysis of milk composition features: Highlighting the predictive power of lactose and electrical conductivity. J. Dairy Res. 2018, 85, 193–200. [Google Scholar] [CrossRef]
- Novichkova, S.; Egorov, S.; Daraselia, N. MedScan, a natural language processing engine for MEDLINE abstracts. Bioinformatics 2003, 19, 1699–1706. [Google Scholar] [CrossRef] [Green Version]
- Nikitin, A.; Egorov, S.; Daraselia, N.; Mazo, I. Pathway studio--the analysis and navigation of molecular networks. Bioinformatics 2003, 19, 2155–2157. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi-Dehcheshmeh, M.; Moghbeli, S.M.; Rahimirad, S.; Alanazi, I.O.; Shehri, Z.S.A.; Ebrahimie, E. A transcription regulatory sequence in the 5′ untranslated region of SARS-CoV-2 Is vital for virus replication with an altered evolutionary pattern against human inhibitory microRNAs. Cells 2021, 10, 319. [Google Scholar] [CrossRef]
- Alanazi, I.O.; AlYahya, S.A.; Ebrahimie, E.; Mohammadi-Dehcheshmeh, M. Computational systems biology analysis of biomarkers in lung cancer; unravelling genomic regions which frequently encode biomarkers, enriched pathways, and new candidates. Gene 2018, 659, 29–36. [Google Scholar] [CrossRef]
- Pashaei-Asl, R.; Pashaei-Asl, F.; Mostafa Gharabaghi, P.; Khodadadi, K.; Ebrahimi, M.; Ebrahimie, E.; Pashaiasl, M. The inhibitory effect of ginger extract on ovarian cancer cell line; Application of systems biology. Adv. Pharm. Bull. 2017, 7, 241–249. [Google Scholar] [CrossRef]
- Ebrahimie, E.; Nurollah, Z.; Ebrahimi, M.; Hemmatzadeh, F.; Ignjatovic, J. Unique ability of pandemic influenza to downregulate the genes involved in neuronal disorders. Mol. Biol. Rep. 2015, 42, 1377–1390. [Google Scholar] [CrossRef]
- Pashaiasl, M.; Ebrahimi, M.; Ebrahimie, E. Identification of the key regulating genes of diminished ovarian reserve (DOR) by network and gene ontology analysis. Mol. Biol. Rep. 2016, 43, 923–937. [Google Scholar] [CrossRef]
- Bakhtiarizadeh, M.R.; Moradi-Shahrbabak, M.; Ebrahimie, E. Underlying functional genomics of fat deposition in adipose tissue. Gene 2013, 521, 122–128. [Google Scholar] [CrossRef]
- Alanazi, I.; Ebrahimie, E.; Hoffmann, P.; Adelson, D.L. Combined gene expression and proteomic analysis of EGF induced apoptosis in A431 cells suggests multiple pathways trigger apoptosis. Apoptosis 2013, 18, 1291–1305. [Google Scholar] [CrossRef]
- Hosseinpour, B.; Bakhtiarizadeh, M.R.; Khosravi, P.; Ebrahimie, E. Predicting distinct organization of transcription factor binding sites on the promoter regions: A new genome-based approach to expand human embryonic stem cell regulatory network. Gene 2013, 531, 212–219. [Google Scholar] [CrossRef]
- Hosseinpour, B.; HajiHoseini, V.; Kashfi, R.; Ebrahimie, E.; Hemmatzadeh, F. Protein interaction network of Arabidopsis thaliana female gametophyte development identifies novel proteins and relations. PLoS ONE 2012, 7, e49931. [Google Scholar] [CrossRef] [Green Version]
- Jahng, J.W.; Kim, J.G.; Kim, H.J.; Kim, B.T.; Kang, D.W.; Lee, J.H. Chronic food restriction in young rats results in depression- and anxiety-like behaviors with decreased expression of serotonin reuptake transporter. Brain Res. 2007, 1150, 100–107. [Google Scholar] [CrossRef]
- File, S.E.; Zangrossi, H., Jr. “One-trial tolerance” to the anxiolytic actions of benzodiazepines in the elevated plus-maze, or the development of a phobic state? Psychopharmacology 1993, 110, 240–244. [Google Scholar] [CrossRef]
- Schneider, P.; Ho, Y.J.; Spanagel, R.; Pawlak, C.R. A novel elevated plus-maze procedure to avoid the one-trial tolerance problem. Front. Behav. Neurosci. 2011, 5, 43. [Google Scholar] [CrossRef] [Green Version]
- Calhoon, G.G.; Tye, K.M. Resolving the neural circuits of anxiety. Nat. Neurosci. 2015, 18, 1394–1404. [Google Scholar] [CrossRef]
- Grillon, C.; Baas, J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin. Neurophysiol. 2003, 114, 1557–1579. [Google Scholar] [CrossRef]
- Plappert, C.F.; Pilz, P.K. Difference in anxiety and sensitization of the acoustic startle response between the two inbred mouse strains BALB/cAN and DBA/2N. Genes Brain Behav. 2002, 1, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Igwe, O.; Sone, M.; Matveychuk, D.; Baker, G.B.; Dursun, S.M. A review of effects of calorie restriction and fasting with potential relevance to depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 111, 110206. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, M. An evolutionary life history framework for psychopathology. Psychol. Inq. 2014, 25, 261–300. [Google Scholar] [CrossRef]
- Ransohoff, J.D.; Wei, Y.; Khavari, P.A. The functions and unique features of long intergenic non-coding RNA. Nat. Rev. Mol. Cell Biol. 2018, 19, 143–157. [Google Scholar] [CrossRef]
- Bai, G.; Qiao, Y.; Lo, P.C.; Song, L.; Yang, Y.; Duan, L.; Wei, S.; Li, M.; Huang, S.; Zhang, B.; et al. Anti-depressive effects of Jiao-Tai-Wan on CORT-induced depression in mice by inhibiting inflammation and microglia activation. J. Ethnopharmacol. 2022, 283, 114717. [Google Scholar] [CrossRef]
- Lehmann, M.L.; Weigel, T.K.; Poffenberger, C.N.; Herkenham, M. The behavioral sequelae of social defeat require microglia and are driven by oxidative stress in mice. J. Neurosci. 2019, 39, 5594–5605. [Google Scholar] [CrossRef] [Green Version]
- McKim, D.B.; Weber, M.D.; Niraula, A.; Sawicki, C.M.; Liu, X.; Jarrett, B.L.; Ramirez-Chan, K.; Wang, Y.; Roeth, R.M.; Sucaldito, A.D.; et al. Microglial recruitment of IL-1beta-producing monocytes to brain endothelium causes stress-induced anxiety. Mol. Psychiatry 2018, 23, 1421–1431. [Google Scholar] [CrossRef] [Green Version]
- Rosin, J.M.; Vora, S.R.; Kurrasch, D.M. Depletion of embryonic microglia using the CSF1R inhibitor PLX5622 has adverse sex-specific effects on mice, including accelerated weight gain, hyperactivity and anxiolytic-like behaviour. Brain Behav. Immun. 2018, 73, 682–697. [Google Scholar] [CrossRef]
- Birt, I.A.; Hagenauer, M.H.; Clinton, S.M.; Aydin, C.; Blandino, P., Jr.; Stead, J.D.H.; Hilde, K.L.; Meng, F.; Thompson, R.C.; Khalil, H.; et al. Genetic liability for internalizing versus externalizing behavior manifests in the developing and adult hippocampus: Insight from a meta-analysis of transcriptional profiling studies in a selectively bred rat model. Biol. Psychiatry 2021, 89, 339–355. [Google Scholar] [CrossRef]
- Maras, P.M.; Hebda-Bauer, E.K.; Hagenauer, M.H.; Hilde, K.L.; Blandino, P., Jr.; Watson, S.J., Jr.; Akil, H. Differences in microglia morphological profiles reflect divergent emotional temperaments: Insights from a selective breeding model. Transl. Psychiatry 2022, 12, 105. [Google Scholar] [CrossRef]
- Zhu, H.; Meissner, L.E.; Byrnes, C.; Tuymetova, G.; Tifft, C.J.; Proia, R.L. The complement regulator Susd4 influences nervous-system function and neuronal morphology in mice. iScience 2020, 23, 100957. [Google Scholar] [CrossRef]
- MacDonald, L.; Hazi, A.; Paolini, A.G.; Kent, S. Calorie restriction dose-dependently abates lipopolysaccharide-induced fever, sickness behavior, and circulating interleukin-6 while increasing corticosterone. Brain Behav. Immun. 2014, 40, 18–26. [Google Scholar] [CrossRef]
- Radler, M.E.; Hale, M.W.; Kent, S. Calorie restriction attenuates lipopolysaccharide (LPS)-induced microglial activation in discrete regions of the hypothalamus and the subfornical organ. Brain Behav. Immun. 2014, 38, 13–24. [Google Scholar] [CrossRef]
- Michopoulos, V.; Powers, A.; Gillespie, C.F.; Ressler, K.J.; Jovanovic, T. Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology 2017, 42, 254–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koponen, E.; Voikar, V.; Riekki, R.; Saarelainen, T.; Rauramaa, T.; Rauvala, H.; Taira, T.; Castren, E. Transgenic mice overexpressing the full-length neurotrophin receptor trkB exhibit increased activation of the trkB-PLCgamma pathway, reduced anxiety, and facilitated learning. Mol. Cell. Neurosci. 2004, 26, 166–181. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, S.K.; Kang, W.S.; Chung, J.H.; Kim, J.W. Increased activation of synapsin 1 and mitogen-activated protein kinases/extracellular signal-regulated kinase in the amygdala of maternal separation rats. CNS Neurosci. Ther. 2014, 20, 172–181. [Google Scholar] [CrossRef]




| Category | Sub-Category | Number |
|---|---|---|
| Entities | Proteins | 144,002 |
| Cell Objects, such as extracellular organelle | 632 | |
| Cell process, including ageing | 14,153 | |
| Cells, including cell lines information | 4297 | |
| Clinical parameters, including energy consumption, energy expenditure, energy intake, and fertility | 5452 | |
| Complexes, such as fatty acid beta-oxidation multienzyme | 994 | |
| Diseases, including depression and anxiety | 23,016 | |
| Pathogens | 607 | |
| Small molecules, including drugs | 1,057,758 | |
| Treatments, including stress | 88 | |
| Tissue, such as hypothalamus | 3908 | |
| Functional Classes, such as Fe transporter | 5570 | |
| Genetic Variants | 168,360 | |
| Virus | 25,323 | |
| Tissues, such as lung mucosa | 588 | |
| Total Entities | 1,454,748 | |
| Relations | Binding relations | 1,176,779 |
| Biomarker relations | 155,684 | |
| Cell expression relations | 1,544,338 | |
| Chemical reaction relations | 64,482 | |
| Clinical trial relations | 133,031 | |
| Direct regulation relations | 798,934 | |
| Expression relations | 1,061,638 | |
| Functional associations | 2,120,740 | |
| Genetic change relations | 481,568 | |
| Molsynthesis | 185,956 | |
| Moltransport | 295,541 | |
| Promoter binding | 52,413 | |
| Protein modification relations | 85,659 | |
| Quantitative change relations | 533,337 | |
| Regulation relations | 6,644,356 | |
| State change relations | 174,659 | |
| MicroRNA effects | 71,649 | |
| Total Relations | 15,580,764 |
| Gene Name | Ensembl Identifier | Fold Change | |||
|---|---|---|---|---|---|
| Hypothalamus | Amygdala | Pituitary | Adrenal Glands | ||
| LOC687780 | 20982 | −1.83 *** | −1.81 ** | −1.83 *** | −2.1 *** |
| AC134224.2 | 62155 | 1.44 ** | 3.66 *** | 2.88 *** | 1.46 ** |
| AABR07044397.1 | 50998 | 3.31 * | 7.47 ** | 5.97 *** | 285.24 *** |
| LOC300308_1 | 48230 | 1.72 *** | 4.44 *** | 1.8 ** | 1.64 * |
| C1qa | 12807 | −1.21 | −1.37 ** | −1.75 *** | −1.87 *** |
| Csf1r | 18414 | −1.24 | −1.23 | −1.35 * | −1.84 *** |
| Gh1 | 11207 | 3.29 *** | −2.03 ** | −1.51 *** | −1.24 |
| Snhg11 | 36802 | 1.39 | 3.73 *** | 2.06 *** | 1.31 |
| Mt-co3 | 30700 | −1.33 | −1.25 | −1.87 *** | −1.5 *** |
| Cacng8 | 57848 | 1.36 * | 2.17 *** | −1.48 | 3.86 * |
| Unc13a | 18452 | 1.26 | 1.45 *** | 2.26 *** | 1.28 |
| AABR07056156.1 | 61013 | 1.47 * | 3.26 *** | 1.63 | 1.37 |
| Abcc5 | 29178 | 1.26 | 2.45 *** | 1.97 *** | 1.14 |
| Lpin3 | 16636 | 1.31 | 4.16 *** | 1.53 *** | 1.13 |
| Mbp | 16516 | −1.37 | −1.57 ** | −3.17 *** | 1.13 |
| AABR07033720.1 | 61472 | 2.56 *** | 2.61 * | 1.42 | −1.72 |
| Nr4a2 | 05600 | 1.19 | −1.22 | −1.53 * | 3.23 *** |
| Cfd | 33564 | −1.34 | −2.31 | −2.93 | −2.52 *** |
| Sppl2a | 11652 | −2.01 *** | −1.08 | −1.5 ** | −1.45 |
| Mcc_1 | 62232 | −92.32 *** | 1.15 | NE | NE |
| Myorg | 23208 | −1.52 *** | −1.5 * | −1.43 | 1.06 |
| Plk5 | 34102 | 1.57 | 1.95 ** | 2.14 *** | 1.12 |
| Rpp30 | 18718 | 1.11 | 3.14 ** | 1.64 ** | 1.14 |
| Bpifa1 | 13859 | 1.98 | 3.86 | 62.13 *** | −2.87 |
| L3mbtl1 | 07044 | 1.52 * | 5.24 *** | 2.61 *** | −1.02 |
| AABR07065531.26 | 59660 | 1.22 | 4.38 *** | 1.63 * | −1.11 |
| Scx | 21812 | 8.03 ** | 7.59 | −1.09 | 11.05 *** |
| Ints10 | 55331 | 1.02 | 2.58 *** | 1.49 *** | 1.2 |
| Mobp | 18700 | −1.3 | −1.45 * | −16.55 *** | 1.01 |
| Tspoap1 | 07957 | 1.04 | 1.15 | 1.96 *** | 1.42 |
| Tmem125_2 | 45872 | −6.98 *** | 1.75 | 1.14 | NE |
| Slc17a7 | 20650 | 3.89 *** | 1.4 | 1.07 | −4.61 |
| AABR07000411.1 | 55789 | 3.63 *** | 2.31 | 1.17 | 1.07 |
| Nadsyn1 | 20736 | 1.14 | 1.23 | 1.95 *** | 1.1 |
| Adamts18 | 11575 | 2.79 *** | −1.03 | 3.35 | 1.34 |
| Acsm5 | 31211 | 1.08 | 1.02 | 1.25 | −7.91 *** |
| Ddn | 59605 | 1.13 | 1.12 | −16.74 *** | 1.02 |
| Cwc25 | 04091 | 1.04 | 1.02 | 1.09 | 3.17 *** |
| Cd300c2 | 46216 | 1.06 | −1.02 | −1.13 | −2.7 *** |
| LOC690507 | 15637 | NE | NE | NE | 9.5 *** |
| Rank | Attribute | Gene Name | Weight | Sum of Weights | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Info Gain Ratio | Rule | Chi Squared | Gini Index | Uncertainty | Relief | Info Gain | ||||
| 1 | ENSRNOG00000019544 | Zbtb2 | 1.0 | 0.8 | 0.9 | 1.0 | 0.9 | 0.6 | 1.0 | 6.1 |
| 2 | ENSRNOG00000062155 | AC134224.2 | 0.8 | 0.5 | 0.9 | 0.9 | 0.8 | 0.9 | 0.8 | 5.6 |
| 3 | ENSRNOG00000051490 | Plcg1 | 0.6 | 1.0 | 0.8 | 0.6 | 0.7 | 0.9 | 0.6 | 5.2 |
| 4 | ENSRNOG00000002194 | Coq2 | 0.9 | 0.6 | 0.7 | 0.9 | 0.7 | 0.6 | 0.9 | 5.1 |
| 5 | ENSRNOG00000009990 | Zranb2 | 0.8 | 0.3 | 0.8 | 0.8 | 0.8 | 1.0 | 0.8 | 5.1 |
| 6 | ENSRNOG00000000975 | Mcoln1 | 0.6 | 0.6 | 1.0 | 0.6 | 0.9 | 0.8 | 0.6 | 5.1 |
| 7 | ENSRNOG00000021061 | Map4k2 | 0.6 | 1.0 | 0.8 | 0.6 | 0.7 | 0.8 | 0.6 | 5.0 |
| 8 | ENSRNOG00000042258 | RGD1561157 | 0.9 | 0.6 | 0.9 | 0.7 | 0.9 | 0.4 | 0.7 | 5.0 |
| 9 | ENSRNOG00000030721 | Fbrsl1 | 0.7 | 0.4 | 0.7 | 0.8 | 0.7 | 0.9 | 0.7 | 4.9 |
| 10 | ENSRNOG00000053405 | Rpap3 | 0.7 | 0.2 | 0.9 | 0.7 | 0.9 | 0.8 | 0.7 | 4.9 |
| 11 | ENSRNOG00000001010 | Tecpr1 | 0.7 | 1.0 | 0.7 | 0.5 | 0.6 | 0.7 | 0.6 | 4.8 |
| 12 | ENSRNOG00000057284 | Cenpb | 0.8 | 1.0 | 0.8 | 0.6 | 0.7 | 0.3 | 0.6 | 4.8 |
| 13 | ENSRNOG00000052539 | Prpf40b | 0.6 | 1.0 | 0.8 | 0.6 | 0.7 | 0.5 | 0.6 | 4.7 |
| 14 | ENSRNOG00000017621 | Spns1 | 0.7 | 0.4 | 0.7 | 0.8 | 0.6 | 1.0 | 0.7 | 4.7 |
| 15 | ENSRNOG00000025711 | Spout1 | 0.6 | 1.0 | 0.7 | 0.6 | 0.6 | 0.7 | 0.5 | 4.7 |
| 16 | ENSRNOG00000020791 | Ube2q1 | 0.8 | 1.0 | 0.8 | 0.6 | 0.8 | 0.1 | 0.6 | 4.7 |
| 17 | ENSRNOG00000010732 | RGD1561590 | 0.7 | 1.0 | 0.7 | 0.5 | 0.7 | 0.5 | 0.6 | 4.7 |
| 18 | ENSRNOG00000042195 | Pabpn1 | 0.7 | 0.4 | 0.8 | 0.6 | 0.7 | 1.0 | 0.5 | 4.6 |
| 19 | ENSRNOG00000014044 | Pank4 | 0.7 | 0.5 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 4.6 |
| 20 | ENSRNOG00000012807 | C1qa | 0.6 | 0.9 | 0.8 | 0.6 | 0.7 | 0.5 | 0.5 | 4.6 |
| 6246 | Region/Tissue | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.7 | 0.0 | 0.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govic, A.; Nasser, H.; Levay, E.A.; Zelko, M.; Ebrahimie, E.; Mohammadi Dehcheshmeh, M.; Kent, S.; Penman, J.; Hazi, A. Long-Term Calorie Restriction Alters Anxiety-like Behaviour and the Brain and Adrenal Gland Transcriptomes of the Ageing Male Rat. Nutrients 2022, 14, 4670. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14214670
Govic A, Nasser H, Levay EA, Zelko M, Ebrahimie E, Mohammadi Dehcheshmeh M, Kent S, Penman J, Hazi A. Long-Term Calorie Restriction Alters Anxiety-like Behaviour and the Brain and Adrenal Gland Transcriptomes of the Ageing Male Rat. Nutrients. 2022; 14(21):4670. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14214670
Chicago/Turabian StyleGovic, Antonina, Helen Nasser, Elizabeth A. Levay, Matt Zelko, Esmaeil Ebrahimie, Manijeh Mohammadi Dehcheshmeh, Stephen Kent, Jim Penman, and Agnes Hazi. 2022. "Long-Term Calorie Restriction Alters Anxiety-like Behaviour and the Brain and Adrenal Gland Transcriptomes of the Ageing Male Rat" Nutrients 14, no. 21: 4670. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14214670