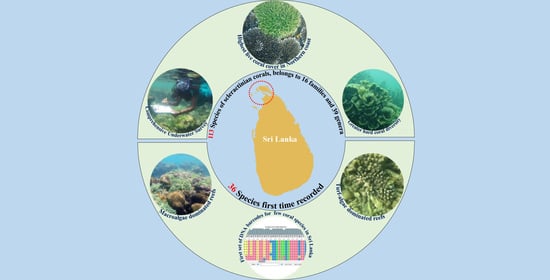
The Status of the Coral Reefs of the Jaffna Peninsula (Northern Sri Lanka), with 36 Coral Species New to Sri Lanka Confirmed by DNA Bar-Coding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
Study Sites
2.2. Surveys and Sampling
2.3. Coral Reef Cover Analysis
2.4. Diversity of Coral Species
2.5. DNA Extraction, Amplification, Sequencing, and Barcoding of Coral Species
2.6. Ethics Statement
3. Results
3.1. Benthic Substrate, Coral Distribution, and the Status of Reefs
3.2. Diversity, Taxonomic Composition, and Distribution of Coral Species
3.3. DNA Barcoding of Selected Coral Species
4. Discussion
4.1. Status of Reefs and Extent of Impacts
4.2. Coral Species Diversity
4.3. DNA Confirmation of Selected Coral Species
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kitahara, M.V.; Fukami, H.; Benzoni, F.; Huang, D. The New Systematics of Scleractinia: Integrating Molecular and Morphological Evidence. In The Cnidaria, Past, Present and Future: The World of Medusa and Her Sisters; Goffredo, S., Dubinsky, Z., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 41–59. ISBN 978-3-319-31305-4. [Google Scholar]
- Harrison, P.L. Sexual Reproduction of Scleractinian Corals. In Coral Reefs: An Ecosystem in Transition; Dubinsky, Z., Stambler, N., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 59–85. ISBN 978-94-007-0114-4. [Google Scholar]
- LaJeunesse, T.C.; Parkinson, J.E.; Gabrielson, P.W.; Jeong, H.J.; Reimer, J.D.; Voolstra, C.R.; Santos, S.R. Systematic Revision of Symbiodiniaceae Highlights the Antiquity and Diversity of Coral Endosymbionts. Curr. Biol. 2018, 28, 2570–2580.e6. [Google Scholar] [CrossRef] [Green Version]
- Muller-Parker, G.; D’Elia, C.F.; Cook, C.B. Interactions between Corals and Their Symbiotic Algae. In Coral Reefs in the Anthropocene; Birkeland, C., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 99–116. ISBN 978-94-017-7249-5. [Google Scholar]
- Reaka-Kudla, M.L.; Wilson, D.E.; Wilson, E.O. Biodiversity II: Understanding and Protecting Our Biological Resources; Joseph Henry Press: Washington, DC, USA, 1996; ISBN 978-0-309-52075-1. [Google Scholar]
- Eakin, C.M.; Sweatman, H.P.A.; Brainard, R.E. The 2014–2017 Global-Scale Coral Bleaching Event: Insights and Impacts. Coral Reefs 2019, 38, 539–545. [Google Scholar] [CrossRef] [Green Version]
- Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; Heron, S.F.; Kerry, J.T.; Lough, J.M.; Baird, A.H.; Baum, J.K.; Berumen, M.L.; Bridge, T.C.; et al. Spatial and Temporal Patterns of Mass Bleaching of Corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.I.L.; Katupotha, J.; Amarasinghe, O.; Manthrithilake, H.; Ariyaratne, B.R. Lagoons of Sri Lanka: From the Origins to the Present; International Water Management Institute: Anand, India, 2013; ISBN 978-92-9090-777-0. [Google Scholar]
- Rajasuriya, A. Provisional Checklist of Corals in SL. In The National Red List 2012 of SL Conservation Status of the Fauna and Flora; Weerakoon, D.K., Wijesundara, S., Eds.; Ministry of Environment: Colombo, Sri Lanka, 2012; pp. 411–430. ISBN 978-955-0033-55-3. [Google Scholar]
- Rajasuriya, A.; White, A.T. Coral Reefs of Sri Lanka: Review of Their Extent, Condition, and Management Status. Coast. Manag. 1995, 23, 77–90. [Google Scholar] [CrossRef]
- Rajasuriya, A. Coral reefs of Sri Lanka: Current status and resource Management. In Regional Workshop on the Conservation and Sustainable Management of Coral Reefs; National Aquatic Resources Research and Development Agency: Colombo, Sri Lanka, 1997. [Google Scholar]
- Ridley, S.O. XXXIV.—The Coral-Fauna of Ceylon, with Descriptions of New Species. Ann. Mag. Nat. Hist. 1883, 11, 250–262. [Google Scholar] [CrossRef]
- Ortmann, A. Beobachtungen an Steinkorallen von der Südküste Ceylons [Observations on the Stone Corals of the South Coast of Sri Lanka]. Zool. J. 1889, 4, 493–590. [Google Scholar]
- Bourne, G.C. Report on the Solitary Corals Collected by Professor Herdman at Ceylon in 1902; Rep. Govt. Ceylon Pearl Oyster Fish: Gulf Mannar, India, 1905; Volume 29, pp. 187–242. [Google Scholar]
- Pillai, C.S.G. Stony Corals of the Seas around India. In Proceedings of the First International Symposium on Corals and Coral Reefs; Marine Biological Association of India: Mandapam, India, 1969. [Google Scholar]
- Mergner, H.; Scheer, G. The Physiographic Zonation and the Ecological Conditions of Some South Indian and Ceylon Reefs. In Proceedings of the 2nd International Coral Reef Symposium; Great Barrier Reef Committee: Brisbane, Australia, 1974; Volume 2, pp. 3–30. [Google Scholar]
- De Silva, M.W.R.N. Status of coral reefs of Sri Lanka, Singapore and Malaysia. In Coral Reef Newsletter; International Union for the Conservation of Nature: Grand, Switzerland, 1981; Volume 3, pp. 34–37. [Google Scholar]
- Scheer, G. The Distribution of Reef-Corals in the Indian Ocean with a Historical Review of Its Investigation. Deep-Sea Res. 1984, 31, 885–900. [Google Scholar] [CrossRef]
- De Silva, M.W.R.N. Status of the coral reefs of Sri Lanka. In Proceedings of the Fifth International Coral Reef Congress, Tahiti, Polynesia, 27 May–1 June 1985; Volume 6, pp. 515–518. [Google Scholar]
- Rajasuriya, A.; De Silva, M.W.R.N. Stony corals of fringing reefs of the Western, South-Western and Southern coasts of Sri Lanka. In Proceedings of the 6th International Coral Reef Symposium, Townsville, Australia, 8–12 August 1988; Volume 3. [Google Scholar]
- Rajasuriya, A. Status of Coral Reefs in the Northern, Western and Southern Coastal Waters of SL. Ten Years after bleaching-facing the consequences of climate change in the Indian Ocean. In CORDIO Status Report; Obura, D., Tamelander, J., Linden, O., Eds.; CORDIO/Sida-SAREC: Mombasa, Kenya, 2008; pp. 11–22. [Google Scholar]
- Kularatne, R.K.A. Suitability of the Coastal Waters of Sri Lanka for Offshore Sand Mining: A Case Study on Environmental Considerations. J. Coast. Conserv. 2014, 18, 227–247. [Google Scholar] [CrossRef]
- Arachchige, G.M.; Jayakody, S.; Mooi, R.; Kroh, A. A Review of Previous Studies on the Sri Lankan Echinoid Fauna, with an Updated Species List. Zootaxa 2017, 4231, zootaxa.4231.2.1. [Google Scholar] [CrossRef]
- Weerakoon, D.; Goonatilake, S.D.A.; Wijewickrama, T.; Rajasuriya, A.; Perera, N.; Kumara, T.P.; De Silva, G.; Miththapala, S.; Mallawatantri, A. Conservation and Sustainable Use of Biodiversity in the Islands and Lagoons of Northern Sri Lanka; IUCN, International Union for Conservation of Nature: Grand, Switzerland, 2020; ISBN 978-2-8317-2088-3. [Google Scholar]
- Dodangodage, P.K. Illegal Fishing by Indian Trawlers Violating the Maritime Boundary of Sri Lanka and Its Impact on Livelihood and the Indo-Sri Lanka Relations. In Proceedings of the 10th International Research Conference of KDU, Ratmalana, Sri Lanka, 3–4 August 2017. [Google Scholar]
- Wijesundara, S.; Amunugama, D. Bottom Trawling in Palk Bay Area: Human and Environmental Implications. In Proceedings of the 10th International Research Conference of KDU, Ratmalana, Sri Lanka, 3–4 August 2017. [Google Scholar]
- Rajasuriya, A. Coral reefs in the Palk Strait and Palk Bay in 2005. J. Natl. Aquat. Resour. Res. Dev. Agency 2007, 38, 77–86. [Google Scholar]
- Rajasuriya, A. Status report on the reef conditions of coral reefs in Sri Lanka. In Coral Reef Degradation in the Indian Ocean Status Report; Lindén, O., Souter, D., Wilhelmsson, D., Obura, D., Eds.; CORDIO, Dept. of Biology and Environmental Science, University of Kalmar: Kalmar, Sweden, 2002. [Google Scholar]
- Sachithananthan, K.; Perera, W.K.T. Topography and substratum of the Jaffna Lagoon. Bull. Fish. Res. Stn. Ceylon 1970, 21, 75–85. [Google Scholar]
- Todd, P.A. Morphological Plasticity in Scleractinian Corals. Biol. Rev. 2008, 83, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Souter, P. Hidden Genetic Diversity in a Key Model Species of Coral. Mar. Biol. 2010, 157, 875–885. [Google Scholar] [CrossRef]
- Veron, J.E.N. Coral Taxonomy and Evolution. In Coral Reefs: An Ecosystem in Transition; Dubinsky, Z., Stambler, N., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 37–45. ISBN 978-94-007-0114-4. [Google Scholar]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; de Waard, J.R. Biological Identifications through DNA Barcodes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitahara, M.V.; Cairns, S.D.; Stolarski, J.; Blair, D.; Miller, D.J.A. Comprehensive Phylogenetic Analysis of the Scleractinia (Cnidaria, Anthozoa) Based on Mitochondrial CO1 Sequence Data. PLoS ONE 2010, 5, e11490. [Google Scholar] [CrossRef] [PubMed]
- Benzoni, F.; Stefani, F.; Pichon, M.; Galli, P. The name game: Morpho-molecular species boundaries in the genus Psammocora (Cnidaria, Scleractinia). Zool. J. Linn. Soc. 2010, 160, 421–456. [Google Scholar] [CrossRef] [Green Version]
- Benzoni, F.; Arrigoni, R.; Waheed, Z.; Stefani, F.; Hoeksema, B. Phylogenetic Relationships and Revision of the Genus Blastomussa (Cnidaria: Anthozoa: Scleractinia) with Description of a New Species. Raffles Bull. Zool. 2014, 62, 358–378. [Google Scholar]
- Gittenberger, A.; Reijnen, B.T.; Hoeksema, B.W. A Molecularly Based Phylogeny Reconstruction of Mushroom Corals (Scleractinia: Fungiidae) with Taxonomic Consequences and Evolutionary Implications for Life History Traits. Contrib. Zool. 2011, 80, 107–132. [Google Scholar] [CrossRef] [Green Version]
- Cooray, P.G. An Introduction to the Geology of Sri Lanka (Ceylon); National Museums of Sri Lanka Publication: Colombo, Sri Lanka, 1984.
- Hodgson, G.; Hill, J.; Kiene, W.; Maun, L.; Mihaly, J.; Liebeler, J.; Shuman, C.; Torres, R. Reef Check Instruction Manual: A Guide to Reef Check Coral Reef Monitoring; Reef Check Foundation: Pacific Palisades, CA, USA, 2006; ISBN 0-9723051-1-4. [Google Scholar]
- Hammer, O.; Harper, D.; Ryan, P. Paleontological Statistics Software Package for Education and Data Analsis. Palaeontol. Electron. 2001, 4, 9–18. [Google Scholar]
- Gomez, E.D.; Aliño, P.M.; Yap, H.T.; Licuanan, W.Y. A review of the status of Philippine reefs. Mar. Pollut. Bull. 1994, 29, 62–68. [Google Scholar] [CrossRef]
- Kelley, R. Indo Pacific Coral Finder, 3rd ed.; BYO Guides: Townsville, Australia, 2016; ISBN 978-0-646-52326-2. [Google Scholar]
- Veron, J.E.N. Corals of the World: Vol 1–3; Australian Institute of Marine Science: Townsville, MC, Australia, 2000; ISBN 978-0-642-32236-4.
- Thukral, A.K.; Bhardwaj, R.; Kumar, V.; Sharma, A. Corrigendum to “New Indices Regarding the Dominance and Diversity of Communities, Derived from Sample Variance and Standard Deviation” [Heliyon 5 (10) (October 2019) E02606]. Heliyon 2019, 5, e03017. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Okonechnikov, K.; Golosova, O.; Fursov, M. UGENE team Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bold Systems V4. Available online: https://v4.boldsystems.org/ (accessed on 10 April 2020).
- Chan, C.-L.; Chen, C.A. Multiplex Next Generation Sequencing of Scleractinian Mitochondrial Genomes. Sequence Submitted (25-JUL-2013) Biodiversity Research Center, Academia Sinica, 128 Academia Road Section 2, Nangang District, Taipei 115, Taiwan. National Center for Biotechnology Information. Acropora aspera mitochondrion, complete genome. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/nuccore/KF448532.1 (accessed on 12 July 2021).
- Robert, R.; Rodrigues, K.F.; Waheed, Z.; Kumar, S.V. Extensive Sharing of Mitochondrial COI and CYB Haplotypes among Reef-Building Staghorn Corals (Acropora Spp.) in Sabah, North Borneo. Mitochondrial DNA Part A 2019, 30, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.A.M.; Ahmed, M.I.; Madkour, F.F.; Hanafy, M.H. Comparative Molecular Ecology Studies on some Scleractinia (Cnidaria, Anthozoa), in the Arabian Gulf and the Egyptian Coast of the Red Sea. Sequence Submitted (01-MAY-2015) Marine Science Department, Faculty of Science, Port Said University, 23 December, Port Said 42526, Egypt. National Center for Biotechnology Information. Acropora digitifera isolate ADS cytochrome oxidase subunit 1 (COI) gene, partial cds; mitochondrial. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/nuccore/KR401100.1 (accessed on 12 July 2021).
- Wijayanti, D.P.; Indrayanti, E.; Nuryadi, H.; Aini, S.N.; Rintiantoto, S.A. Genetic Diversity of Two Coral Species, Pocillopora damicornis and Acropora hyacinthus from Wakatobi waters, Indonesia. Submitted (04-OCT-2017) Contact:Diah Permata Wijayanti Diponegoro, University, Marine Science Department; Prof. Soedarto, SH. Semarang, Central Java 50275, Indonesia URL:http://www.fpik.undip.ac.id. National Center for Biotechnology Information. Acropora hyacinthus Mitochondrial H_Ah17 Gene for Cytochrome Oxidase Subunit I, Partial cds. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/nuccore/LC326547.1 (accessed on 12 July 2021).
- Arrigoni, R.; Stefani, F.; Pichon, M.; Galli, P.; Benzoni, F. Molecular Phylogeny of the Robust Clade (Faviidae, Mussidae, Merulinidae, and Pectiniidae): An Indian Ocean Perspective. Mol. Phylogenet. Evol. 2012, 65, 183–193. [Google Scholar] [CrossRef]
- Forsman, Z.H.; Concepcion, G.T.; Haverkort, R.D.; Shaw, R.W.; Maragos, J.E.; Toonen, R.J. Ecomorph or Endangered Coral? DNA and Microstructure Reveal Hawaiian Species Complexes: Montipora dilatata/flabellata/turgescens & M. patula/verrilli. PLoS ONE 2010, 5, e15021. [Google Scholar] [CrossRef] [Green Version]
- Venkataraman, K.; Satyanarayan, C.; Alfred, J.R.B.; Wolstenholme, J. Hand Book on Hard Corals of India; Director, Zoological Survey of India: Kolkata, India, 2003; ISBN 81-8171-20-7.
- Bahuguna, A.; Chaudhury, R.N.; Bhattji, N.; Navalgund, R.R. Spatial Inventory and Ecological Status of Coral Reefs of the Central Indian Ocean Using Resourcesat-1. Indian J. Geo-Mar. Sci. 2013, 42, 684–696. [Google Scholar]
- Arthur, R. Coral Bleaching and Mortality in Three Indian Reef Regions during an El Niño Southern Oscillation Event. Curr. Sci. 2000, 79, 1723–1729. [Google Scholar]
- Rajasuriya, A.; Zahir, H.; Muley, E.V.; Subramanian, B.R.; Venkataraman, K.; Wafar, S.M.; Hannan Khan, M.; Whittingham, E. Status of Coral Reefs in South Asia: Bangladesh, India, Maldives and Sri Lanka. In Status of Coral Reefs of the World; Wilkinson, C., Ed.; Australian Institute of Marine Science: Townsville, Australia, 2000; pp. 95–115. [Google Scholar]
- Patterson, E.; Gilbert, M.; Raj, K.D.; Thinesh, T.; Patterson, J.; Tamelander, J. Coral Reefs of Gulf of Mannar, India -Signs of Resilience. In Proceedings of the 12th International Coral Reef Symposium, Cairns, Australia, 9–13 July 2012. [Google Scholar]
- Arthur, R. Patterns of Benthic recovery in Lakshadweep atolls. In Ten Years after Bleaching–Facing the Consequences of Climate Change in the Indian Ocean CORDIO Status Report; Obura, D.O., Tamelander, J., Linden, O., Eds.; Coastal Oceans Research and Development in the Indian Ocean/Sida–SAREC: Mombasa, Kenya, 2008; pp. 39–44. [Google Scholar]
- Krishnan, P.; Purvaja, R.; Sreeraj, C.R.; Raghuraman, R.; Robin, R.S.; Abhilash, K.R.; Mahendra, R.S.; Anand, A.; Gopi, M.; Mohanty, P.C.; et al. Differential Bleaching Patterns in Corals of Palk Bay and the Gulf of Mannar. Curr. Sci. 2018, 114, 679. [Google Scholar] [CrossRef]
- Manikandan, B.; Ravindran, J. Differential Response of Coral Communities to Caulerpa Spp. Bloom in the Reefs of Indian Ocean. Environ. Sci. Pollut. Res. 2017, 24, 3912–3922. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.; Satyanarayan, C.; Raghunathan, C.; Koya, S.S.; Ravindran, J.; Manikandan, B.; Venkataraman, K. Status and Review of Health of Indian Coral Reefs. J. Aquat. Biol. Fish. 2015, 3, 1–14. [Google Scholar]
- Marimuthu, N.; Yogesh Kumar, J.S.; Raghunathan, C.; Vinithkumar, N.V.; Kirubagaran, R.; Sivakumar, K.; Venkataraman, K. North-South Gradient of Incidence, Distribution and Variations of Coral Reef Communities in the Andaman and Nicobar Islands, India. J. Coast. Conserv. 2017, 21, 289–301. [Google Scholar] [CrossRef]
- Machendiranathan, M.; Ranith, R.; Senthilnathan, L.; Saravanakumar, A.; Thangaradjou, T. Resilience of Coral Recruits in Gulf of Mannar Marine Biosphere Reserve (GOMMBR), India. Reg. Stud. Mar. Sci. 2020, 34, 101055. [Google Scholar] [CrossRef]
- Sukumaran, S.; George, R.M.; Kasinathan, C. Community Structure and Spatial Patterns of Hard Coral Biodiversity in Kilakarai Group of Islands in Gulf of Mannar, India. J. Mar. Biol. Assoc. India 2008, 50, 79–86. [Google Scholar]
- Krishnan, P.; Dam Roy, S.; George, G.; Srivastava, R.C.; Anand, A.; Murugesan, S.; Kaliyamoorthy, M.; Vikas, N.; Soundararajan, R. Elevated sea surface temperature during May 2010 induces mass bleaching of corals in the Andaman. Curr. Sci. 2011, 100, 111. [Google Scholar]
- Sheppard, C.; Harris, A.; Sheppard, A. Archipelago-Wide Coral Recovery Patterns since 1998 in the Chagos Archipelago, Central Indian Ocean. Mar. Ecol. Prog. Ser. 2008, 362, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.D.; Gilmour, J.P.; Heyward, A.J. Resilience of Coral Communities on an Isolated System of Reefs Following Catastrophic Mass-Bleaching. Coral Reefs 2008, 27, 197–205. [Google Scholar] [CrossRef]
- Rajasuriya, A.; Karunaratne, C. Post-bleaching status of the coral reefs of Sri Lanka. Coral reef degradation in the Indian Ocean. In Coral Reef Degradation in the Indian Ocean: Status Report 2000; Souter, D., Obura, D., Linden, O., Eds.; CORDIO, SAREC Marine Science Program, Stockholm University: Stockholm, Sweden, 2000; pp. 54–63. [Google Scholar]
- Rajasuriya, A. Status of coral reefs in Sri Lanka in the aftermath of the 1998 coral bleaching event and 2004 tsunami. In Coral Reef Degradation in the Indian Ocean: Status Report 2005; Souter, D., Linden, O., Eds.; CORDIO, Department of Biology and Environmental Science, University of Kalmar: Småland, Sweden, 2005; pp. 83–96. [Google Scholar]
- Rajasuriya, A.; Perera, N.; Fernando, M. Status of coral reefs in Trincomalee, Sri Lanka. In Coral Reef Degradation in the Indian Ocean: Status Report 2005; Souter, D., Lindėn, O., Eds.; CORDIO, Department of Biology and Environmental Science, University of Kalmar: Småland, Sweden, 2005; pp. 97–103. [Google Scholar]
- Kularatne, R.K.A. Unregulated and Illegal Fishing by Foreign Fishing Boats in Sri Lankan Waters with Special Reference to Bottom Trawling in Northern Sri Lanka: A Critical Analysis of the Sri Lankan Legislation. Ocean Coast. Manag. 2020, 185, 105012. [Google Scholar] [CrossRef]
- Survey Department, Statistics Unit, Ministry of Fisheries and Aquatic Resources Development. 2018. Available online: https://www.fisheriesdept.gov.lk/web/images/pdf/Fisheries_Statistics_2018.pdf (accessed on 19 May 2019).
- Hoeksema, B.W.; Cairns, S. World List of Scleractinia. 2021. Available online: http://www.marinespecies.org/scleractinia (accessed on 18 April 2021).
- Venkataraman, K.; Rajan, R.; Satyanarayana, C.H.; Raghunathan, C.; Venkatraman, C. Marine Ecosystems and Marine Protected Areas of India; Director, Zoological Survey of India: Kolkata, India, 2012; ISBN 978-81-8171-312-4. [Google Scholar]
- Raghuraman, R.; Sreeraj, C.R.; Raghunathan, C.; Venkataraman, K. Scleractinian coral diversity in Andaman Nicobar Island in comparison with other Indian reefs. In International Day for Biological Diversity Marine Biodiversity 2012; Uttar Pradesh State Biodiversity Board: Uttar Pradesh, India, 2012; pp. 75–92. [Google Scholar]
- Venkataraman, K.; Rajan, R. Status of Coral Reefs in Palk Bay. Rec. Zool. Surv. India 2013, 113, 1–11. [Google Scholar]
- Loya, Y.; Sakai, K.; Yamazato, K.; Nakano, Y.; Sambali, H.; van Woesik, R. Coral Bleaching: The Winners and the Losers. Ecol. Lett. 2001, 4, 122–131. [Google Scholar] [CrossRef]
- Woesik, R.; van Sakai, K.; Ganase, A.; Loya, Y. Revisiting the winners and the losers a decade after coral bleaching. Mar. Ecol. Prog. Ser. 2011, 434, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.K.; Robinson, J.P.W.; Chong-Seng, K.; Robinson, J.; Graham, N.A.J. Boom and bust of keystone structure on coral reefs. Coral Reefs 2019, 38, 625–635. [Google Scholar] [CrossRef]
- Lough, J.M. Small change, big difference: Sea surface temperature distributions for tropical coral reef ecosystems, 1950–2011. J. Geophys Res. Ocean. 2012, 117. [Google Scholar] [CrossRef] [Green Version]
- Polónia, A.R.M.; Cleary, D.F.R.; de Voogd, N.J.; Renema, W.; Hoeksema, B.W.; Martins, A.; Gomes, N.C.M. Habitat and Water Quality Variables as Predictors of Community Composition in an Indonesian Coral Reef: A Multi-Taxon Study in the Spermonde Archipelago. Sci. Total Environ. 2015, 537, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Gunarathna, A.B.A.K.; Rajasuriya, A. Extent and status of coral reefs in northern coastal waters of Sri Lanka. In Proceedings of the 25th Anniversary Scientific Conference of NARA on Tropical Aquatic Research towards Sustainable Development, Colombo, Sri Lanka, 15–16 February 2007; p. 41. [Google Scholar]
- Veron, J.E.N.; Stafford-Smith, M.; DeVantier, L.; Turak, E. Overview of distribution patterns of zooxanthellate Scleractinia. Front. Mar. Sci. 2015, 1. [Google Scholar] [CrossRef] [Green Version]
- Evans, N.; Paulay, G. DNA Barcoding Methods for Invertebrates. In DNA Barcodes: Methods and Protocols; Kress, W.J., Erickson, D.L., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; pp. 47–77. ISBN 978-1-61779-591-6. [Google Scholar]
- Duncan, E.J.; Gluckman, P.D.; Dearden, P.K. Epigenetics, plasticity, and evolution: How do we link epigenetic change to phenotype? J. Exp. Zool. B Mol. Dev. Evol. 2014, 322, 208–220. [Google Scholar] [CrossRef] [Green Version]







| No. | Family | Jaffna | |
|---|---|---|---|
| Genera | Species | ||
| 1 | Acroporidae Verrill, 1902 | 4 | 28 |
| 2 | Agariciidae Gray, 1847 | 2 | 3 |
| 3 | Dendrophylliidae Gray, 1847 | 1 | 5 |
| 4 | Diploastraeidae Chevalier & Beauvais, 1987 | 1 | 1 |
| 5 | Euphylliidae Alloiteau, 1952 | 1 | 2 |
| 6 | Fungiidae Dana, 1846 | 2 | 3 |
| 7 | Merulinidae Verrill, 1866 | 12 | 39 |
| 8 | Leptastreidae Rowlett, 2020 | 2 | 3 |
| 9 | Lobophylliidae Dai & Horng, 2009 | 5 | 8 |
| 10 | Oulastreidae Vaughan, 1919 | 1 | 1 |
| 11 | Plesiastreidae Dai & Horng, 2009 | 1 | 1 |
| 12 | Pocilloporidae Gray, 1842 | 2 | 3 |
| 13 | Poritidae Gray, 1842 | 2 | 13 |
| 14 | Psammocoridae Chevalier & Beauvais, 1987 | 1 | 1 |
| 15 | Siderastreidae Vaughan & Wells, 1943 | 1 | 2 |
| 16 | Scleractinia incertae sedis (temporary name) | 1 | 1 |
| Total | 39 | 113 | |
| Ptm | Inb | Tho | Val | Knr | Pu1, Pu2 | Kt1, Kt2 | Del | |
|---|---|---|---|---|---|---|---|---|
| Families | 12 | 07 | 12 | 08 | 09 | 06 | 08 | 10 |
| Genera | 24 | 27 | 24 | 18 | 17 | 15 | 18 | 18 |
| Species | 49 | 30 | 44 | 25 | 36 | 34 | 33 | 37 |
| No. | Family | Genus | Species | PS Sites | PB Sites | LWI | GOM | ANI | AS | CIO |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Acroporidae Verrill, 1901 | Acropora Oken, 1815 | Acropora aspera Dana, 1846 | √ | √ | √ | √ | √ | ||
| 2 | Acropora digitiferaDana, 1846 | √ | √ | √ | √ | √ | √ | |||
| 3 | Acropora gemmifera Brook, 1892 | √ | √ | √ | √ | √ | ||||
| 4 | Acropora latistella Brook, 1891 | √ | √ | √ | √ | |||||
| 5 | Acropora pulchra Brook, 1891 | √ | √ | √ | √ | |||||
| 6 | Alveopora Blainville, 1830 | Alveopora allingi Hoffmeister, 1925 | √ | √ | √ | √ | ||||
| 7 | Astreopora Blainville, 1830 | Astreopora myriophthalma Lamarck, 1816 | √ | √ | √ | √ | √ | √ | √ | |
| 8 | Astreopora listeri Bernard, 1896 | √ | √ | √ | √ | √ | ||||
| 9 | Astreopora ocellata Bernard, 1896 | √ | √ | √ | ||||||
| 10 | Montipora Blainville, 1830 | Montipora flabellata Studer,1901 | √ | √ | ||||||
| 11 | Montipora informis Bernard, 1897 | √ | √ | √ | √ | √ | √ | √ | ||
| 12 | Merulinidae Verrill, 1865 | Goniastrea Milne Edwards & Haime, 1848 | Goniastrea minuta Veron, 2000 | √ | √ | √ | ||||
| 13 | Coelastrea Verrill, 1866 | Coelastrea palauensis Yabe & Sugiyama, 1936 | √ | √ | ||||||
| 14 | Cyphastrea Milne Edwards & Haime, 1848 | Cyphastrea japonica Yabe & Sugiyama, 1936 | √ | √ | ||||||
| 15 | Cyphastrea microphthalma Lamarck, 1816 | √ | √ | √ | √ | √ | √ | |||
| 16 | Platygyra Ehrenberg, 1834 | Platygyra acuta Veron, 2000 | √ | √ | √ | √ | ||||
| 17 | Echinopora Lamarck, 1816 | Echinopora gemmacea Lamarck, 1816 | √ | √ | √ | √ | ||||
| 18 | Dipsastraea Blainville, 1830 | Dipsastraea amicorum Milne Edwards & Haime, 1850 | √ | √ | √ | |||||
| 19 | Dipsastraea lizardensis Veron, Pichon & Wijsman-Best, 1977 | √ | √ | √ | √ | |||||
| 20 | Dipsastraea rotumana Gardiner, 1899 | √ | √ | √ | √ | |||||
| 21 | Scleractinia incertae sedis | Pachyseris Milne Edwards & Haime, 1849 | Pachyseris gemmae Nemenzo, 1955 | √ | √ | √ | ||||
| 22 | Oulastreidae Vaughan, 1919 | Oulastrea Milne Edwards & Haime, 1848 | Oulastrea crispata Lamarck, 1816 | √ | √ | √ | ||||
| 23 | Poritidae Gray,1842 | Porites Link, 1807 | Porites evermanni Vaughan, 1907 | √ | √ | √ | ||||
| 24 | Porites murrayensis Vaughan, 1918 | √ | √ | √ | √ | |||||
| 25 | Porites pukoensis Vaughan, 1907 | √ | √ | |||||||
| 26 | Goniopora de Blainville, 1830 | Goniopora lobata Milne Edwards, 1860 | √ | √ | √ | √ | ||||
| 27 | Goniopora minor Crossland, 1952 | √ | √ | √ | √ | √ | √ | |||
| 28 | Goniopora somaliensis Vaughan, 1907 | √ | √ | √ | ||||||
| 29 | Goniopora tenuidens Quelch, 1886 | √ | √ | √ | √ | |||||
| 30 | Dendrophylliidae Gray, 1847 | Turbinaria Oken, 1815 | Turbinaria frondens Dana, 1846 | √ | √ | |||||
| 31 | Turbinaria reniformis Bernard, 1896 | √ | √ | √ | √ | |||||
| 32 | Turbinaria stelluta Lamarck, 1816 | √ | √ | √ | √ | √ | ||||
| 33 | Lobophylliidae Dai & Horng, 2009 | Acanthastrea Milne Edwards & Haime, 1848 | Acanthastrea ishigakiensis Veron, 1990 | √ | √ | √ | ||||
| 34 | Micromussa Veron, 2000 | Micromussa amakusensis Veron, 1990 | √ | |||||||
| 35 | Agariciidae Gray, 1847 | Coeloseris Vaughan, 1918 | Coeloseris mayeri Vaughan, 1918 | √ | √ | √ | ||||
| 36 | Siderastreidae Vaughan & Wells, 1943 | Siderastrea Blainville, 1830 | Siderastrea savignyana Milne Edwards & Haime, 1849 | √ | √ | √ | √ | √ |
| Reef Regions | Number of Species | Richness | Shannon’s Diversity Index (H’) | Simpson’s Dominance Index (D) | Simpson’s Diversity Index (D’) | Evenness Index (E) |
|---|---|---|---|---|---|---|
| Point Pedro | 49 | 3.429 | 2.927 | 0.066 | 0.934 | 0.921 |
| Inbarsitty | 30 | 2.921 | 2.61 | 0.084 | 0.916 | 0.900 |
| Thondaimanaru | 44 | 3.769 | 3.02 | 0.060 | 0.94 | 0.938 |
| Valithoondal | 25 | 3.470 | 2.752 | 0.069 | 0.931 | 0.971 |
| Karainagar | 35 | 2.704 | 2.575 | 0.089 | 0.911 | 0.929 |
| Pungudutivu | 32 | 2.475 | 2.323 | 0.127 | 0.873 | 0.880 |
| Kayts | 34 | 3.258 | 2.743 | 0.083 | 0.917 | 0.932 |
| Delft | 37 | 2.959 | 2.713 | 0.082 | 0.918 | 0.939 |
| Sample ID | Accession ID | Sequence Length | Molecular Identification (Closest Relative in GenBank) | Identity | Reference |
|---|---|---|---|---|---|
| COJP001 | MN689059 | 709 | Acropora aspera (KF448532.1) | 99.69% | [48] |
| COJP002 | MN689060 | 717 | Acropora gemmifera (MG383839.1) | 99.83% | [49] |
| COJP003 | MN689061 | 721 | Acropora digitifera (KR401100.1) | 99.22% | [50] |
| COJP004 | MN689062 | 711 | Acropora gemmifera (MG383839.1) | 99.83% | [49] |
| COJP006 | MN689067 | 715 | Acropora hyacinthus (LC326547.1) | 99.85% | [51] |
| COJP007 | MN689068 | 718 | Echinopora gemmacea (HE654561.1) | 99.84% | [52] |
| COJP009 | MN689065 | 714 | Montipora flabellata (HQ246603.1) | 99.51% | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arulananthan, A.; Herath, V.; Kuganathan, S.; Upasanta, A.; Harishchandra, A. The Status of the Coral Reefs of the Jaffna Peninsula (Northern Sri Lanka), with 36 Coral Species New to Sri Lanka Confirmed by DNA Bar-Coding. Oceans 2021, 2, 509-529. https://0-doi-org.brum.beds.ac.uk/10.3390/oceans2030029
Arulananthan A, Herath V, Kuganathan S, Upasanta A, Harishchandra A. The Status of the Coral Reefs of the Jaffna Peninsula (Northern Sri Lanka), with 36 Coral Species New to Sri Lanka Confirmed by DNA Bar-Coding. Oceans. 2021; 2(3):509-529. https://0-doi-org.brum.beds.ac.uk/10.3390/oceans2030029
Chicago/Turabian StyleArulananthan, Ashani, Venura Herath, Sivashanthini Kuganathan, Anura Upasanta, and Akila Harishchandra. 2021. "The Status of the Coral Reefs of the Jaffna Peninsula (Northern Sri Lanka), with 36 Coral Species New to Sri Lanka Confirmed by DNA Bar-Coding" Oceans 2, no. 3: 509-529. https://0-doi-org.brum.beds.ac.uk/10.3390/oceans2030029