Decrease of Antimicrobial Resistance through Polyelectrolyte-Coated Nanoliposomes Loaded with β-Lactam Drug
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Liposome Systems
2.2.1. Experimental Design Optimization
2.2.2. Preparation of Liposomes Loaded with ampicillin
2.2.3. Liposome Surface Modification
2.3. Characterization of Liposome Systems
2.3.1. Zeta Potential and Size Measurements
2.3.2. Encapsulation Efficiency
2.4. Stability of Liposomes
2.5. Antimicrobial Susceptibility Test
3. Results and Discussion
3.1. Optimization of Liposome Preparation Process
3.2. Liposome Surface Modification
3.3. Stability of Liposome
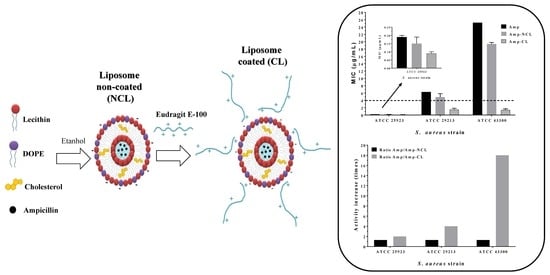
3.4. Antimicrobial Susceptibility Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Brooks, B.D.; Brooks, A.E. Therapeutic strategies to combat antibiotic resistance. Adv. Drug Deliv. Rev. 2014, 78, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2017, 3099, 1–10. [Google Scholar] [CrossRef]
- Pinto-Alphandary, H.; Andremont, A.; Couvreur, P. Targeted delivery of antibiotics using liposomes and nanoparticles: Research and applications. Int. J. Antimicrob. Agents 2000, 13, 155–168. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Suleman, N.; Mocktar, C.; Seedat, N.; Govender, T. Nanoengineered drug delivery systems for enhancing antibiotic therapy. J. Pharm. Sci. 2015, 104, 872–905. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, F.P.; Cruciani, M. Worldwide Epidemiology and Antibiotic Resistance of Staphylococcus aureus. In Staphylococcus Aureus; Fabio, B., Rino, R., Guido, G., Eds.; Springer: New York, NY, USA, 2016; pp. 21–56. [Google Scholar]
- Yılmaz, E.Ş.; Aslantaş, Ö. Antimicrobial resistance and underlying mechanisms in Staphylococcus aureus isolates. Asian Pac. J. Trop. Med. 2017, 10, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, T.; Myc, A.; Donovan, B.; Shih, A.Y.; Reuter, J.D.; Baker, J.R. A novel surfactant nanoemulsion with a unique non-irritant topical antimicrobial activity against bacteria, enveloped viruses and fungi. Microbiol. Res. 2001, 156, 1–7. [Google Scholar] [CrossRef] [PubMed]
- LiPuma, J.J.; Rathinavelu, S.; Foster, B.K.; Keoleian, J.C.; Makidon, P.E.; Kalikin, L.M.; Baker, J.R. In vitro activities of a novel nanoemulsion against Burkholderia and other multidrug-resistant cystic fibrosis-associated bacterial species. Antimicrob. Agents Chemother. 2009, 53, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.Y.; Ramalingam, K.; Bienek, D.R.; Lee, V.; You, T.; Alvarez, R. Antimicrobial activity of nanoemulsion in combination with cetylpyridinium chloride in multidrug-resistant acinetobacter baumannii. Antimicrob. Agents Chemother. 2013, 57, 3568–3575. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, C.H.; Yarce, C.J.; Roman, Y.; Davalos, A.F.; Rivera, G.R. Application of Nanoparticle Technology to Reduce the Anti-Microbial Resistance through β-Lactam Antibiotic-Polymer Inclusion Nano-Complex. Pharmaceuticals 2018, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, R.; Valacchi, G.; Muresan, X.M.; Drechsler, M.; Contado, C.; Esposito, E.; Grandini, A.; Guerrini, A.; Forlani, G.; Sacchetti, G. Nanostructured lipid carriers (NLC) for the delivery of natural molecules with antimicrobial activity: Production, characterisation and in vitro studies. J. Microencapsul. 2017, 34, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, R.; Leonardi, A.; Fuochi, V.; Petronio, G.P.; Greco, A.S.; Furneri, P.M. A method for efficient loading of ciprofloxacin hydrochloride in cationic solid lipid nanoparticles: Formulation and microbiological evaluation. Nanomaterials 2018, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Calabretta, M.K.; Kumar, A.; McDermott, A.M.; Cai, C. Antibacterial activities of poly(amidoamine) dendrimers terminated with amino and poly(ethylene glycol) groups. Biomacromolecules 2007, 8, 1807–1811. [Google Scholar] [CrossRef] [PubMed]
- Scorciapino, M.A.; Serra, I.; Manzo, G.; Rinaldi, A.C. Antimicrobial Dendrimeric Peptides: Structure, Activity and New Therapeutic Applications. Int. J. Mol. Sci. 2017, 18, 542. [Google Scholar] [CrossRef] [PubMed]
- Çağdaş, M.; Sezer, A.D.; Bucak, S. Liposomes as Potential Drug Carrier Systems for Drug Delivery. In Application of Nanotechnology in Drug Delivery; InTech: Vienna, Austria, 2014. [Google Scholar] [Green Version]
- Maherani, B.; Arab-Tehrany, E.; Mozafari, M.R.; Gaiani, C.; Linder, M. Liposomes: A Review of Manufacturing Techniques and Targeting Strategies. Curr. Nanosci. 2011, 7, 436–452. [Google Scholar] [CrossRef]
- Eloy, J.O.; Claro de Souza, M.; Petrilli, R.; Barcellos, J.P.A.; Lee, R.J.; Marchetti, J.M. Liposomes as carriers of hydrophilic small molecule drugs: Strategies to enhance encapsulation and delivery. Colloids Surfaces B Biointerfaces 2014, 123, 345–363. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, S.K. In vitro release kinetics model fitting of liposomes: An insight. Chem. Phys. Lipids 2016, 201, 28–40. [Google Scholar] [CrossRef]
- Drulis-Kawa, Z.; Dorotkiewicz-Jach, A. Liposomes as delivery systems for antibiotics. Int. J. Pharm. 2010, 387, 187–198. [Google Scholar] [CrossRef]
- Marianecci, C.; Petralito, S.; Rinaldi, F.; Hanieh, P.N.; Carafa, M. Some recent advances on liposomal and niosomal vesicular carriers. J. Drug Deliv. Sci. Technol. 2016, 32, 256–269. [Google Scholar] [CrossRef]
- Bangale, G.S.; Rajesh, K.S.; Shinde, G. V Stealth Liposomes: A Novel Approach of Targeted Drug Delivery in Cancer Therapy. Int. J. Pharma Sci. Res. 2014, 5, 750–759. [Google Scholar]
- Nag, O.K.; Awasthi, V. Surface engineering of liposomes for stealth behavior. Pharmaceutics 2013, 5, 542–569. [Google Scholar] [CrossRef] [PubMed]
- Arenas Fernández, T.; Mora Arango, C.L.; Salamanca, C.H.; Jaramillo Flórez, M.C. Actividad del (2E)-3-(2, 3-dimetoxifenil)-1-(4-metilfenil) prop-2-en-1-ona en presencia del poli(ácido maleico-co-2-vinil-pirrolidona) sobre un aislamiento clínico de Staphylococcus aureus productor de β-lactamasas TT-Activity of (2E)-3-(2, 3-dimetoxif. Iatreia 2012, 25, 12–19. [Google Scholar]
- Ukawa, M.; Akita, H.; Hayashi, Y.; Ishiba, R.; Tange, K.; Arai, M.; Kubo, K.; Higuchi, Y.; Shimizu, K.; Konishi, S.; et al. Neutralized nanoparticle composed of SS-cleavable and pH-activated lipid-like material as a long-lasting and liver-specific gene delivery system. Adv. Healthc. Mater. 2014, 3, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement; CLSI: Wayne, PA, USA, 2015; ISBN 1562387855. [Google Scholar]
- Aniansson, E.A.G.; Wall, S.N.; Almgren, M.; Hoffman, H.; Kielman, I.; Ulbricht, W.; Zana, R.; Lang, J.; Tondre, C. Theory of the kinetics of micellar equilibria and quantitative interpretation of chemical relaxation studies of micelar solutions of ionic surfactants. J. Phys. Chem. 1976, 80, 905–922. [Google Scholar] [CrossRef]
- Carrión, F.J.; De la Maza, A.; Parra, J.L. La influencia de la fuerza iónica y la carga de la bicapa lipídica en la estabilidad de liposomas. J. Colloid Interface Sci. 1994, 164, 78–87. [Google Scholar] [CrossRef]
- Sabín, J.; Prieto, G.; Ruso, J.M.; Hidalgo-Álvarez, R.; Sarmiento, F. Size and stability of liposomes: A possible role of hydration and osmotic forces. Eur. Phys. J. E 2006, 20, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Yandrapati, R.K. Effect of Lipid Composition on the Physical Properties of Liposomes: A Light Scattering Study; Missouri University of Science and Technology: Rolla, MO, USA, 2012. [Google Scholar]
- Kotyńska, J.; Figaszewski, Z.A. Adsorption equilibria between liposome membrane formed of phosphatidylcholine and aqueous sodium chloride solution as a function of pH. Biochim. Biophys. Acta Biomembr. 2005, 1720, 22–27. [Google Scholar] [CrossRef]
- Alasino, R.V.; Leonhard, V.; Bianco, I.D.; Beltramo, D.M. Eudragit E100 surface activity and lipid interactions. Colloids Surfaces B Biointerfaces 2012, 91, 84–89. [Google Scholar] [CrossRef]
- Hasanovic, A.; Hollick, C.; Fischinger, K.; Valenta, C. Improvement in physicochemical parameters of DPPC liposomes and increase in skin permeation of aciclovir and minoxidil by the addition of cationic polymers. Eur. J. Pharm. Biopharm. 2010, 75, 148–153. [Google Scholar] [CrossRef]
- Grema, H.A. Methicillin Resistant Staphylococcus aureus (MRSA): A Review. Adv. Anim. Vet. Sci. 2015, 3, 79–98. [Google Scholar] [CrossRef] [Green Version]
- Stapleton, P.D.; Taylor, P.W. Methicillin resistance in Staphylococcus aureus: Mechanisms and modulation. Sci. Prog. 2002, 85, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.D.; Taylor, P.W. Methicillin reistance in staphylococcus aureus. Lancet 1970, 295, 800–804. [Google Scholar]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef] [PubMed]





| Run | Ionic Strength (mM) | Cut-Off (MWCO) | Aging Time (min) | Run | Ionic Strength (mM) | Cut-Off (MWCO) | Aging Time (min) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 30 kDa | 5 | 19 | 10 | 10 kDa | 20 |
| 2 | 0 | 30 kDa | 20 | 20 | 10 | 30 kDa | 5 |
| 3 | 1 | 10 kDa | 5 | 21 | 10 | 30 kDa | 5 |
| 4 | 1 | 30 kDa | 20 | 22 | 0 | 10 kDa | 20 |
| 5 | 10 | 10 kDa | 5 | 23 | 10 | 30 kDa | 20 |
| 6 | 1 | 10 kDa | 20 | 24 | 10 | 10 kDa | 20 |
| 7 | 10 | 10 kDa | 5 | 25 | 1 | 10 kDa | 5 |
| 8 | 10 | 10 kDa | 5 | 26 | 1 | 30 kDa | 20 |
| 9 | 0 | 30 kDa | 5 | 27 | 0 | 10 kDa | 5 |
| 10 | 1 | 30 kDa | 20 | 28 | 1 | 10 kDa | 20 |
| 11 | 10 | 10 kDa | 20 | 29 | 1 | 10 kDa | 20 |
| 12 | 0 | 30 kDa | 5 | 30 | 0 | 10 kDa | 5 |
| 13 | 10 | 30 kDa | 20 | 31 | 0 | 30 kDa | 5 |
| 14 | 1 | 30 kDa | 5 | 32 | 10 | 30 kDa | 20 |
| 15 | 10 | 30 kDa | 5 | 33 | 1 | 30 kDa | 5 |
| 16 | 1 | 10 kDa | 5 | 34 | 0 | 10 kDa | 20 |
| 17 | 0 | 10 kDa | 20 | 35 | 0 | 30 kDa | 20 |
| 18 | 0 | 10 kDa | 5 | 36 | 0 | 30 kDa | 20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arévalo, L.M.; Yarce, C.J.; Oñate-Garzón, J.; Salamanca, C.H. Decrease of Antimicrobial Resistance through Polyelectrolyte-Coated Nanoliposomes Loaded with β-Lactam Drug. Pharmaceuticals 2019, 12, 1. https://0-doi-org.brum.beds.ac.uk/10.3390/ph12010001
Arévalo LM, Yarce CJ, Oñate-Garzón J, Salamanca CH. Decrease of Antimicrobial Resistance through Polyelectrolyte-Coated Nanoliposomes Loaded with β-Lactam Drug. Pharmaceuticals. 2019; 12(1):1. https://0-doi-org.brum.beds.ac.uk/10.3390/ph12010001
Chicago/Turabian StyleArévalo, Lina M., Cristhian J. Yarce, José Oñate-Garzón, and Constain H. Salamanca. 2019. "Decrease of Antimicrobial Resistance through Polyelectrolyte-Coated Nanoliposomes Loaded with β-Lactam Drug" Pharmaceuticals 12, no. 1: 1. https://0-doi-org.brum.beds.ac.uk/10.3390/ph12010001