Lipid-Lowering Bioactivity of Microalga Nitzschia laevis Extract Containing Fucoxanthin in Murine Model and Carcinomic Hepatocytes
Abstract
:1. Introduction
2. Results
2.1. NLE Composition and Fucoxanthin Identification
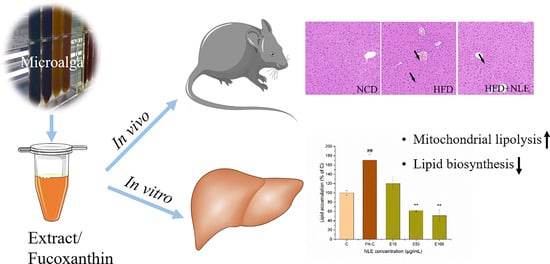
2.2. NLE Prevented Bodyweight Gain and Lipid Accumulation in HFD-Treated Mice
2.3. NLE Attenuated Lipid Accumulation in HepG2 Cells
2.4. NLE Increased Mitochondrial Fatty Acids Metabolism in HepG2 Cells
2.5. NLE Increased Mitochondrial Membrane Potential of HepG2 Cells
2.6. NLE Inhibited Fatty Acid Synthesis in HepG2 Cells
2.7. Fucoxanthin Prevented Hepatic Lipid Accumulation in PA-Treated HepG2 Cells
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation of Nitzschia Laevis Extract and Identification of Fucoxanthin
4.3. Animals and Treatments
4.4. Histological and Biochemical Analysis
4.5. Cell Culture and Treatments
4.6. Cytotoxicity Assay
4.7. Oil Red O Staining and Quantification
4.8. Mitochondrial Respiration Assay
4.9. Mitochondrial Membrane Potential Analysis
4.10. mRNA Extraction and Quantitative Real-time PCR Analysis
4.11. Western Blot Analysis
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, G.; You, H.J.; Bajaj, J.S.; Joo, S.K.; Yu, J.; Park, S.; Kang, H.; Park, J.H.; Kim, J.H.; Lee, D.H.; et al. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Rinella, M.E. Nonalcoholic fatty liver disease: A systematic review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Geng, T.; Zou, Q.; Yang, N.; Zhao, W.; Li, Y.; Tan, X.; Yuan, T.; Liu, X.; Liu, Z. Lycopene prevents lipid accumulation in hepatocytes by stimulating PPARα and improving mitochondrial function. J. Funct. Foods 2020, 67, 103857. [Google Scholar] [CrossRef]
- Sasaki, G.Y.; Li, J.; Cichon, M.J.; Riedl, K.M.; Kopec, R.E.; Bruno, R.S. Green tea extract treatment in obese mice with nonalcoholic steatohepatitis restores the hepatic metabolome in association with limiting endotoxemia-TLR4-NFκB-mediated inflammation. Mol. Nutr. Food Res. 2019, 63, e1900811. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zhuge, F.; Nagashimada, M.; Ota, T. Novel action of carotenoids on non-alcoholic fatty liver disease: Macrophage polarization and liver homeostasis. Nutrients 2016, 8, 391. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Mo, W.; Feng, J.; Li, J.; Yu, Q.; Li, S.; Zhang, J.; Chen, K.; Ji, J.; Dai, W.; et al. Astaxanthin attenuates hepatic damage and mitochondrial dysfunction in non-alcoholic fatty liver disease by up-regulating the FGF21/PGC-1α pathway. Br. J. Pharmacol. 2020, 177, 3760–3777. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Liu, B.; Wei, H.; Cheng, K.W.; Chen, F. Extract of the microalga Nitzschia laevis prevents high-fat-diet-induced obesity in mice by modulating the composition of gut microbiota. Mol. Nutr. Food Res. 2019, 63, 1800808. [Google Scholar] [CrossRef]
- Takatani, N.; Kono, Y.; Beppu, F.; Okamatsu-Ogura, Y.; Yamano, Y.; Miyashita, K.; Hosokawa, M. Fucoxanthin inhibits hepatic oxidative stress, inflammation, and fibrosis in diet-induced nonalcoholic steatohepatitis model mice. Biochem. Biophys. Res. Commun. 2020, 528, 305–310. [Google Scholar] [CrossRef]
- Peng, K.-Y.; Watt, M.J.; Rensen, S.; Greve, J.W.; Huynh, K.; Jayawardana, K.S.; Meikle, P.J.; Meex, R.C.R. Mitochondrial dysfunction-related lipid changes occur in nonalcoholic fatty liver disease progression. J. Lipid Res. 2018, 59, 1977–1986. [Google Scholar] [CrossRef] [Green Version]
- Morio, B.; Panthu, B.; Bassot, A.; Rieusset, J. Role of mitochondria in liver metabolic health and diseases. Cell Calcium 2021, 94, 102336. [Google Scholar] [CrossRef]
- Alkhouri, N.; Lawitz, E.; Noureddin, M.; DeFronzo, R.; Shulman, G.I. Gs-0976 (firsocostat): An investigational liver-directed acetyl-coa carboxylase (ACC) inhibitor for the treatment of non-alcoholic steatohepatitis (NASH). Expert Opin. Investig. Drugs 2020, 29, 135–141. [Google Scholar] [CrossRef]
- Lally, J.S.V.; Ghoshal, S.; DePeralta, D.K.; Moaven, O.; Wei, L.; Masia, R.; Erstad, D.J.; Fujiwara, N.; Leong, V.; Houde, V.P.; et al. Inhibition of acetyl-coa carboxylase by phosphorylation or the inhibitor ND-654 suppresses lipogenesis and hepatocellular carcinoma. Cell Metab. 2019, 29, 174–182.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of NAFLD-related hcc: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2020, 18, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef] [PubMed]
- Abidov, M.; Ramazanov, Z.; Seifulla, R.; Grachev, S. The effects of xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes. Metab. 2010, 12, 72–81. [Google Scholar] [CrossRef]
- Guo, B.; Yang, B.; Pang, X.; Chen, T.; Chen, F.; Cheng, K.-W. Fucoxanthin modulates cecal and fecal microbiota differently based on diet. Food Funct. 2019, 10, 5644–5655. [Google Scholar] [CrossRef]
- Chang, Y.H.; Chen, Y.L.; Huang, W.C.; Liou, C.J. Fucoxanthin attenuates fatty acid-induced lipid accumulation in FL83B hepatocytes through regulated Sirt1/AMPK signaling pathway. Biochem. Biophys. Res. Commun. 2018, 495, 197–203. [Google Scholar] [CrossRef]
- Koliaki, C.; Szendroedi, J.; Kaul, K.; Jelenik, T.; Nowotny, P.; Jankowiak, F.; Herder, C.; Carstensen, M.; Krausch, M.; Knoefel, W.T.; et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015, 21, 739–746. [Google Scholar] [CrossRef] [Green Version]
- Sinton, M.C.; Meseguer-Ripolles, J.; Lucendo-Villarin, B.; Lyall, M.J.; Carter, R.N.; Morton, N.M.; Hay, D.C.; Drake, A.J. Nonalcoholic fatty liver disease is associated with decreased hepatocyte mitochondrial respiration but not mitochondrial number. bioRxiv 2020. [Google Scholar]
- Zeng, X.; Yang, J.; Hu, O.; Huang, J.; Ran, L.; Chen, M.; Zhang, Y.; Zhou, X.; Zhu, J.; Zhang, Q.; et al. Dihydromyricetin ameliorates nonalcoholic fatty liver disease by improving mitochondrial respiratory capacity and redox homeostasis through modulation of SIRT3 signaling. Antioxid. Redox Signal. 2019, 30, 163–183. [Google Scholar] [CrossRef]
- Albracht-Schulte, K.; Gonzalez, S.; Jackson, A.; Wilson, S.; Ramalingam, L.; Kalupahana, N.S.; Moustaid-Moussa, N. Eicosapentaenoic acid improves hepatic metabolism and reduces inflammation independent of obesity in high-fat-fed mice and in HepG2 cells. Nutrients 2019, 11, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2017, 552, 50–59. [Google Scholar] [CrossRef]
- Lei, P.; Tian, S.; Teng, C.; Huang, L.; Liu, X.; Wang, J.; Zhang, Y.; Li, B.; Shan, Y. Sulforaphane improves lipid metabolism by enhancing mitochondrial function and biogenesis in vivo and in vitro. Mol. Nutr. Food Res. 2019, 63, 1800795. [Google Scholar] [CrossRef] [PubMed]
- McGarry, J.D.; Leatherman, G.F.; Foster, D.W. Carnitine palmitoyltransferase i. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J. Biol. Chem. 1978, 253, 4128–4136. [Google Scholar] [CrossRef]
- Guo, B.; Liu, B.; Yang, B.; Sun, P.; Lu, X.; Liu, J.; Chen, F. Screening of diatom strains and characterization of cyclotella cryptica as a potential fucoxanthin producer. Mar. Drugs 2016, 14, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, M.; Maeda, A.; Tani, S.; Akagawa, M. Palmitate induces insulin resistance in human hepg2 hepatocytes by enhancing ubiquitination and proteasomal degradation of key insulin signaling molecules. Arch. Biochem. Biophys. 2015, 566, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Guardia-Escote, L.; Mulero, M.; Basaure, P.; Biosca-Brull, J.; Cabré, M.; Colomina, M.T.; Domingo, J.L.; Sánchez, D.J. Obesogenic effects of chlorpyrifos and its metabolites during the differentiation of 3T3-L1 preadipocytes. Food Chem. Toxicol. 2020, 137, 111171. [Google Scholar] [CrossRef] [PubMed]








| Parameters | Units | NCD | HFD | HFD + NLE |
|---|---|---|---|---|
| CHO | mM | 2.33 ± 0.10 | 4.65 ± 0.47 | 3.39 ± 0.13 |
| TG | mM | 0.19 ± 0.07 | 0.16 ± 0.05 | 0.13 ± 0.05 |
| HDL-C | mM | 1.33 ± 0.04 | 1.72 ± 0.14 | 1.18 ± 0.24 |
| LDL-C | mM | 0.10 ± 0.01 | 0.25 ± 0.03 | 0.2 ± 0.02 |
| ALT | U/L | 25.9 ± 3.18 | 127.9 ± 15.3 | 85.5 ± 9.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, B.; Zhou, Y.; Liu, B.; He, Y.; Chen, F.; Cheng, K.-W. Lipid-Lowering Bioactivity of Microalga Nitzschia laevis Extract Containing Fucoxanthin in Murine Model and Carcinomic Hepatocytes. Pharmaceuticals 2021, 14, 1004. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14101004
Guo B, Zhou Y, Liu B, He Y, Chen F, Cheng K-W. Lipid-Lowering Bioactivity of Microalga Nitzschia laevis Extract Containing Fucoxanthin in Murine Model and Carcinomic Hepatocytes. Pharmaceuticals. 2021; 14(10):1004. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14101004
Chicago/Turabian StyleGuo, Bingbing, Yonghui Zhou, Bin Liu, Yongjin He, Feng Chen, and Ka-Wing Cheng. 2021. "Lipid-Lowering Bioactivity of Microalga Nitzschia laevis Extract Containing Fucoxanthin in Murine Model and Carcinomic Hepatocytes" Pharmaceuticals 14, no. 10: 1004. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14101004