Synthesis and Biological Characterization of the New Glycolipid Lactose Undecylenate (URB1418)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
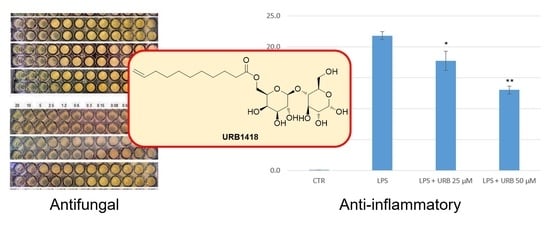
2.2. Antifungal and Antibacterial Activity
2.3. Anti-Inflammatory Properties
2.4. Radical Scavenging Properties
2.5. Evaluation of the Antioxidant Properties
2.6. Evaluation of the Cytotoxic Effects
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of 6′-O-Undec-10-enoyl-4-O-(3′,4′-O-isopropylidene-β-d-galactopyranosyl)-2,3:5,6-di-O-isopropylidene-1,1-di-O-methyl-d-glucopyranose (3, LTA Undecylenate)
3.3. Synthesis of 6′-O-Undec-10-enoyl-4-O-(β-d-galactopyranosyl)-d-glucopyranose (4, Lactose Undecylenate, URB1418)
3.4. Microbes and Culture Conditions
3.5. Minimum Inhibitory Concentration
3.6. DPPH Assays
3.7. DNA Nicking Assays
3.8. Cytotoxicity Assays
3.9. DCFH-DA Assay
3.10. Nitric Oxide Detection
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De, S.; Malik, S.; Ghosh, A.; Saha, R.; Saha, B. A review on natural surfactants. RSC Adv. 2015, 5, 65757–65767. [Google Scholar] [CrossRef]
- Mnif, I.; Ellouz-Chaambouni, S.; Ghribi, D. Glycolipid biosurfactants, main classes, functional properties and related potential applications in environmental biotechnology. J. Polym. Environ. 2018, 26, 2192–2206. [Google Scholar] [CrossRef]
- Staroń, J.; Dąbrowski, J.M.; Cichoń, E.; Guzik, M. Lactose esters: Synthesis and biotechnological applications. Crit. Rev. Biotechnol. 2018, 38, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Verboni, M.; Lucarini, S.; Duranti, A. 6′-O-Lactose esters surfactants as an innovative opportunity in the pharmaceutical field: From synthetic methods to biological applications. Pharmaceuticals 2021, 14, 1306. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, S.; Fagioli, L.; Campana, R.; Cole, H.; Duranti, A.; Baffone, W.; Vllasaliu, D.; Casettari, L. Unsaturated fatty acids lactose esters: Cytotoxicity, permeability enhancement and antimicrobial activity. Eur. J. Pharm. Biopharm. 2016, 107, 88–96. [Google Scholar] [CrossRef]
- Lucarini, S.; Fagioli, L.; Cavanagh, R.; Liang, W.; Perinelli, D.; Campana, M.; Stolnik, S.; Lam, J.; Casettari, L.; Duranti, A. Synthesis, structure–activity relationships and in vitro toxicity profile of lactose-based fatty acid monoesters as possible drug permeability enhancers. Pharmaceutics 2018, 10, 81. [Google Scholar] [CrossRef] [Green Version]
- Perinelli, D.R.; Lucarini, S.; Fagioli, L.; Campana, R.; Vllasaliu, D.; Duranti, A.; Casettari, L. Lactose oleate as new biocompatible surfactant for pharmaceutical applications. Eur. J. Pharm. Biopharm. 2018, 124, 55–62. [Google Scholar] [CrossRef]
- Campana, R.; Merli, A.; Verboni, M.; Biondo, F.; Favi, G.; Duranti, A.; Lucarini, S. Synthesis and evaluation of saccharide-based aliphatic and aromatic esters as antimicrobial and antibiofilm agents. Pharmaceuticals 2019, 12, 186. [Google Scholar] [CrossRef] [Green Version]
- McCartney, F.; Perinelli, D.R.; Tiboni, M.; Cavanagh, R.; Lucarini, S.; Palmieri, G.P.; Casettari, L.; Brayden, D.J. Permeability-enhancing effects of three laurate-disaccharide monoesters across isolated rat intestinal mucosae. Int. J. Pharm. 2021, 601, 120593. [Google Scholar] [CrossRef]
- Tiboni, M.; Elmowafy, E.; El-Derany, M.O.; Benedetti, S.; Campana, R.; Verboni, M.; Potenza, L.; Palma, F.; Citterio, B.; Sisti, M.; et al. A combination of sugar esters and chitosan to promote in vivo wound care. Int. J. Pharm. 2022, 616, 121508. [Google Scholar] [CrossRef]
- Shapiro, A.L.; Rothman, S. Undecylenic acid in the treatment of dermatomycosis. Arch. Dermatol. Syphilol. 1945, 52, 166–171. [Google Scholar] [CrossRef]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Tasneem, S.; Liu, B.; Li, B.; Choudhary, M.I.; Wang, W. Molecular pharmacology of inflammation: Medicinal plants as anti-inflammatory agents. Pharmacol. Res. 2019, 139, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Ahmad Khan, M.S.; Singh Cameotra, S.; Safar Al-Thubiani, A. Biosurfactants: Potential applications as immunomodulator drugs. Immunol. Lett. 2020, 223, 71–77. [Google Scholar] [CrossRef]
- Subramaniam, M.D.; Venkatesan, D.; Iyer, M.; Subbarayan, S.; Govindasami, V.; Roy, A.; Narayanasamy, A.; Kamalakannan, S.; Gopalakrishnan, A.V.; Thangarasu, R.; et al. Biosurfactants and anti-inflammatory activity: A potential new approach towards COVID-19. Curr. Opin. Environ. Sci. Health 2020, 17, 72–81. [Google Scholar] [CrossRef]
- Lucarini, S.; Ciulla, M.G.; Mestichelli, P.; Duranti, A. Total Synthesis of Natural Disaccharide Sambubiose. Pharmaceuticals 2020, 13, 198. [Google Scholar] [CrossRef]
- Pianalto, K.M.; Alspaugh, J.A. New horizons in antifungal therapy. J. Fungi 2016, 2, 26. [Google Scholar] [CrossRef]
- Perfect, J.R. Is there an emerging need for new antifungals? Expert Opin. Emerg. Drugs 2016, 21, 129–131. [Google Scholar] [CrossRef]
- Vassilev, D.; Petkova, N.; Tumbarski, Y.; Koleva, M.; Denev, P. Application of the principles of “green chemistry” for the synthesis of 10-undecylenic aliphatic esters with antimicrobial activity. J. Renew. Mater. 2020, 8, 675–686. [Google Scholar] [CrossRef]
- Shi, D.; Zhao, Y.; Yan, H.; Fu, H.; Shen, Y.; Lu, G.; Mei, H.; Qiu, Y.; Li, D.; Liu, W. Antifungal effects of undecylenic acid on the biofilm formation of Candida albicans. Int. J. Clin. Pharmacol. Ther. 2016, 54, 343–353. [Google Scholar] [CrossRef] [PubMed]
- McLain, N.; Ascanio, R.; Baker, C.; Strohaver, R.A.; Dolan, J.W. Undecylenic acid inhibits morphogenesis of Candida albicans. Antimicrob. Agents Chemother. 2000, 44, 2873–2875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazu, T.K.; Bricker, B.A.; Flores-Rozas, H.; Ablordeppey, S.Y. The mechanistic targets of antifungal agents: An overview. Mini Rev. Med. Chem. 2016, 16, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-M.; Luedecke, L.O.; Swanson, B.G.; Davidson, P.M. Inhibition of microorganisms in salad dressing by sucrose and methylglucose fatty acid monoesters. J. Food Process. Preserv. 2003, 27, 285–298. [Google Scholar] [CrossRef]
- Zhang, X.; Song, F.; Taxipalati, M.; Wei, W.; Feng, F. Comparative study of surface-active properties and antimicrobial activities of disaccharide monoesters. PLoS ONE 2014, 9, e114845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabisz, Ł.; Piotrowicz, Z.; Dąbrowska, M.; Dobrowolska, A.; Czaczyk, K.; Nowak, I.; Łęska, B. Sweet surfactants I: Fatty acid esters of sucralose. Food Chem. 2021, 358, 129827. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef]
- Bluth, M.H.; Kandil, E.; Mueller, C.M.; Shah, V.; Lin, Y.Y.; Zhang, H.; Dresner, L.; Lempert, L.; Nowakowski, M.; Gross, R.; et al. Sophorolipids block lethal effects of septic shock in rats in a cecal ligation and puncture model of experimental sepsis. Crit. Care Med. 2006, 34, 188–195. [Google Scholar] [CrossRef]
- Hwang, M.-H.; Lim, J.-H.; Yun, H.-I.; Rhee, M.-H.; Cho, J.-Y.; Hsu, W.H.; Park, S.-C. Surfactin C inhibits the lipopolysaccharide-induced transcription of interleukin-1beta and inducible nitric oxide synthase and nitric oxide production in murine RAW 264.7 cells. Biotechnol. Lett. 2005, 27, 1605–1608. [Google Scholar] [CrossRef]
- Borio, A.; Holgado, A.; Garate, J.A.; Beyaert, R.; Heine, H.; Zamyatina. A. Disaccharide-based anionic amphiphiles as potent inhibitors of lipopolysaccharide-induced inflammation. ChemMedChem 2018, 13, 2317–2331. [Google Scholar] [CrossRef] [Green Version]
- Zamyatina, A.; Heine, H. Lipopolysaccharide recognition in the crossroads of TLR4 and caspase-4/11 mediated inflammatory pathways. Front. Immunol. 2020, 11, 584146. [Google Scholar] [CrossRef] [PubMed]
- Heine, H.; Adanitsch, F.; Peternelj, T.T.; Haegman, M.; Kasper, C.; Ittig, S.; Beyaert, R.; Jerala, R.; Zamyatina, A. Tailored modulation of cellular pro-inflammatory responses with disaccharide lipid A mimetics. Front. Immunol. 2021, 12, 631797. [Google Scholar] [CrossRef] [PubMed]
- Merghni, A.; Dallel, I.; Noumi, E.; Kadmi, Y.; Hentati, H.; Tobji, S.; Ben Amor, A.; Mastouri, M. Antioxidant and antiproliferative potential of biosurfactants isolated from Lactobacillus casei and their anti-biofilm effect in oral Staphylococcus aureus strains. Microb. Pathog. 2017, 104, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Basit, M.; Rasool, M.H.; Naqvi, S.A.R.; Waseem, M.; Aslam, B. Biosurfactants production potential of native strains of Bacillus cereus and their antimicrobial, cytotoxic and antioxidant activities. Pak. J. Pharm. Sci. 2018, 31 (Suppl. 1), 251–256. [Google Scholar]
- Giri, S.S.; Ryu, E.C.; Sukumaran, V.; Park, S.C. Antioxidant, antibacterial, and anti-adhesive activities of biosurfactants isolated from Bacillus strains. Microb. Pathog. 2019, 132, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Mouafo, H.T.; Mbawala, A.; Somashekar, D.; Tchougang, H.M.; Harohally, N.V.; Ndjouenkeu, R. Biological properties and structural characterization of a novel rhamnolipid like-biosurfactants produced by Lactobacillus casei subsp. casei TM1B. Biotechnol. Appl. Biochem. 2021, 68, 585–596. [Google Scholar] [CrossRef]
- Hough, L.; Richardson, A.C.; Thelwall, L.A.W. Reaction of lactose with 2,2-dimethoxypropane: A tetraacetal of novel structure. Carbohydr. Res. 1979, 75, C11–C12. [Google Scholar] [CrossRef]
- Sarney, D.B.; Kapeller, H.; Fregapane, G.; Vulfson, E.N. Chemo-enzymatic synthesis of disaccharide fatty acid esters. J. Am. Oil Chem. Soc. 1994, 71, 711–714. [Google Scholar] [CrossRef]
- Ricciutelli, M.; Bartolucci, G.; Campana, R.; Salucci, S.; Benedetti, S.; Caprioli, G.; Maggi, F.; Sagratini, G.; Vittori, S.; Lucarini, S. Quantification of 2- and 3-isopropylmalic acids in forty Italian wines by UHPLC-MS/MS triple quadrupole and evaluation of their antimicrobial, antioxidant activities and biocompatibility. Food Chem. 2020, 321, 126726. [Google Scholar] [CrossRef]
- Mari, G.; Catalani, S.; Antonini, E.; de Crescentini, L.; Mantellini, F.; Santeusanio, S.; Lombardi, P.; Amicucci, A.; Battistelli, S.; Benedetti, S.; et al. Synthesis and biological evaluation of novel heteroring-annulated pyrrolino-tetrahydroberberine analogues as antioxidant agents. Bioorg. Med. Chem. 2018, 26, 5037–5044. [Google Scholar] [CrossRef]
- Mari, G.; de Crescentini, L.; Benedetti, S.; Palma, F.; Santeusanio, S.; Mantellini, F. Synthesis of new dihydroberberine and tetrahydroberberine analogues and evaluation of their antiproliferative activity on NCI-H1975 cells. Beilstein J. Org. Chem. 2020, 16, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Tiboni, M.; Benedetti, S.; Skouras, A.; Curzi, G.; Perinelli, D.R.; Palmieri, G.F.; Casettari, L. 3D-printed microfluidic chip for the preparation of glycyrrhetinic acid-loaded ethanolic liposomes. Int. J. Pharm. 2020, 584, 119436. [Google Scholar] [CrossRef] [PubMed]
- Catalani, S.; Palma, F.; Battistelli, S.; Benedetti, S. Oxidative stress and apoptosis induction in human thyroid carcinoma cells exposed to the essential oil from Pistacia lentiscus aerial parts. PLoS ONE 2017, 12, e0172138. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S.; Grisham, M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef] [Green Version]
- Petrović, M.; Bonvin, D.; Hofmann, H.; Mionić Ebersold, M. Fungicidal PMMA-Undecylenic Acid Composites. Int. J. Mol. Sci. 2018, 19, 184. [Google Scholar] [CrossRef] [Green Version]
- Serbina, N.V.; Jia, T.; Hohl, T.M.; Pamer, E.G. Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol. 2008, 26, 421–452. [Google Scholar] [CrossRef] [Green Version]








| MICs (µg/mL) | ||
|---|---|---|
| URB1418 | UA | |
| Trichophyton mentagrophytes F6 | >1024 | 512 |
| Trichophyton rubrum F2 | 512 | 256 |
| Trichophyton violaceum F11 | >1024 | 512 |
| Epidermophyton floccosum F12 | >1024 | 256 |
| C. albicans ATCC 10231 | 512 | 128 |
| MICs (µg/mL) URB1418 | |
|---|---|
| S. aureus HCS026 | >1024 |
| S. aureus HCS002 (MRSA) | >1024 |
| S. aureus 2/5 | >1024 |
| S. aureus 28/10 | >1024 |
| S. aureus 18/9 | >1024 |
| S. aureus MRSA ATCC 43300 | >1024 |
| S. aureus ATCC 43387 | >1024 |
| P. aeruginosa C86 | >1024 |
| P. aeruginosa ATCC 27583 | >1024 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verboni, M.; Benedetti, S.; Campana, R.; Palma, F.; Potenza, L.; Sisti, M.; Duranti, A.; Lucarini, S. Synthesis and Biological Characterization of the New Glycolipid Lactose Undecylenate (URB1418). Pharmaceuticals 2022, 15, 456. https://0-doi-org.brum.beds.ac.uk/10.3390/ph15040456
Verboni M, Benedetti S, Campana R, Palma F, Potenza L, Sisti M, Duranti A, Lucarini S. Synthesis and Biological Characterization of the New Glycolipid Lactose Undecylenate (URB1418). Pharmaceuticals. 2022; 15(4):456. https://0-doi-org.brum.beds.ac.uk/10.3390/ph15040456
Chicago/Turabian StyleVerboni, Michele, Serena Benedetti, Raffaella Campana, Francesco Palma, Lucia Potenza, Maurizio Sisti, Andrea Duranti, and Simone Lucarini. 2022. "Synthesis and Biological Characterization of the New Glycolipid Lactose Undecylenate (URB1418)" Pharmaceuticals 15, no. 4: 456. https://0-doi-org.brum.beds.ac.uk/10.3390/ph15040456