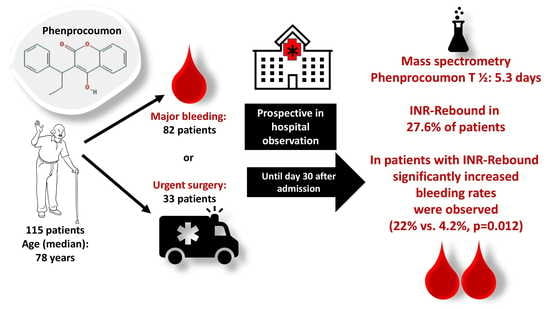
Pharmacokinetics of Phenprocoumon in Emergency Situations–Results of the Prospective Observational RADOA-Registry (Reversal Agent Use in Patients Treated with Direct Oral Anticoagulants or Vitamin K Antagonists Registry)
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Oversight
4.2. Patients
- Age > 18 years.
- Patients anticoagulated with DOACs or phenprocoumon with clinically overt major bleeding according to a modified definition according to the International Society of Thrombosis and Haemostasis for nonsurgical patients [27] that presented with at least one of the following criteria: symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial, or intramuscular with compartment syndrome or acute life-threatening blood loss leading to hemodynamic instability and/or acute transfusion of two or more units of whole blood or red cells.
- Patients anticoagulated with DOACs or phenprocoumon needing an urgent surgical intervention within 24 h after admission.
- Patient inclusion lasted from 2014 to March 2018.
4.3. Ethics
4.4. Substudy of the RADOA-Registry to Analyze Pharmacokinetics of Phenproumon
4.5. UPLC-MS/MS Measurement of Phenprocoumon
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carnicelli, A.P.; Hong, H.; Connolly, S.J.; Eikelboom, J.; Giugliano, R.P.; Morrow, D.A.; Patel, M.R.; Wallentin, L.; Alexander, J.H.; Cecilia Bahit, M.; et al. Direct Oral Anticoagulants Versus Warfarin in Patients with Atrial Fibrillation: Patient-Level Network Meta-Analyses of Randomized Clinical Trials with Interaction Testing by Age and Sex. Circulation 2022, 145, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Kakkos, S.K.; Gohel, M.; Baekgaard, N.; Bauersachs, R.; Bellmunt-Montoya, S.; Black, S.A.; Ten Cate-Hoek, A.J.; Elalamy, I.; Enzmann, F.K.; Geroulakos, G.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2021 Clinical Practice Guidelines on the Management of Venous Thrombosis. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 9–82. [Google Scholar] [CrossRef] [PubMed]
- Cimpan, P.L.; Chira, R.I.; Mocan, M.; Anton, F.P.; Farcas, A.D. Oral Anticoagulant Therapy-When Art Meets Science. J. Clin. Med. 2019, 8, 1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, K.L.; Kunst, M.; Leuchs, A.K.; Bohme, M.; Weckbecker, K.; Kastenmuller, K.; Bleckwenn, M.; Holdenrieder, S.; Coch, C.; Hartmann, G.; et al. Phenprocoumon Dose Requirements, Dose Stability and Time in Therapeutic Range in Elderly Patients with CYP2C9 and VKORC1 Polymorphisms. Front. Pharmacol. 2019, 10, 1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansell, J.; Hirsh, J.; Hylek, E.; Jacobson, A.; Crowther, M.; Palareti, G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008, 133, 160S–198S. [Google Scholar] [CrossRef]
- Merli, G.J.; Tzanis, G. Warfarin: What are the clinical implications of an out-of-range-therapeutic international normalized ratio? J. Thromb. Thrombolysis 2009, 27, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Ufer, M. Comparative pharmacokinetics of vitamin K antagonists: Warfarin, phenprocoumon and acenocoumarol. Clin. Pharmacokinet. 2005, 44, 1227–1246. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.; Cordonnier, C.; Korv, J.; Lal, A.; Ovesen, C.; Purrucker, J.C.; Toni, D.; Steiner, T. European Stroke Organisation Guideline on Reversal of Oral Anticoagulants in Acute Intracerebral Haemorrhage. Eur. Stroke J. 2019, 4, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Parry-Jones, A.R.; Di Napoli, M.; Goldstein, J.N.; Schreuder, F.H.; Tetri, S.; Tatlisumak, T.; Yan, B.; van Nieuwenhuizen, K.M.; Dequatre-Ponchelle, N.; Lee-Archer, M.; et al. Reversal strategies for vitamin K antagonists in acute intracerebral hemorrhage. Ann. Neurol. 2015, 78, 54–62. [Google Scholar] [CrossRef]
- Dentali, F.; Crowther, M.A. Management of excessive anticoagulant effect due to vitamin K antagonists. Hematol. Am. Soc. Hematol. Educ. Program 2008, 2008, 266–270. [Google Scholar] [CrossRef]
- Warkentin, L.; Hueber, S.; Deiters, B.; Klohn, F.; Kuhlein, T. Vitamin-K-antagonist phenprocoumon versus low-dose direct oral anticoagulants (DOACs) in patients with atrial fibrillation: A real-world analysis of German claims data. Thromb. J. 2022, 20, 31. [Google Scholar] [CrossRef]
- Hohnloser, S.H.; Basic, E.; Nabauer, M. Uptake in antithrombotic treatment and its association with stroke incidence in atrial fibrillation: Insights from a large German claims database. Clin. Res. Cardiol. 2019, 108, 1042–1052. [Google Scholar] [CrossRef]
- Altiok, E.; Marx, N. Oral Anticoagulation. Dtsch. Arztebl. Int. 2018, 115, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Lindhoff-Last, E. Direct oral anticoagulants (DOAC)—Management of emergency situations. Hamostaseologie 2017, 37, 257–266. [Google Scholar] [CrossRef]
- Lindhoff-Last, E.; Herrmann, E.; Lindau, S.; Konstantinides, S.; Grottke, O.; Nowak-Goettl, U.; Lucks, J.; Zydek, B.; Heymann, C.V.; Birschmann, I.; et al. Severe Hemorrhage Associated with Oral Anticoagulants. Dtsch. Arztebl. Int. 2020, 117, 312–319. [Google Scholar] [CrossRef]
- Pfeilschifter, W.; Lindhoff-Last, E.; Alhashim, A.; Zydek, B.; Lindau, S.; Konstantinides, S.; Grottke, O.; Nowak-Gottl, U.; von Heymann, C.; Birschmann, I.; et al. Intracranial bleeding under vitamin K antagonists or direct oral anticoagulants: Results of the RADOA registry. Neurol. Res. Pract. 2022, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Lindhoff-Last, E.; Birschmann, I.; Kuhn, J.; Lindau, S.; Konstantinides, S.; Grottke, O.; Nowak-Gottl, U.; Lucks, J.; Zydek, B.; von Heymann, C.; et al. Pharmacokinetics of Direct Oral Anticoagulants in Emergency Situations: Results of the Prospective Observational RADOA-Registry. Thromb. Haemost. 2022, 122, 552–559. [Google Scholar] [CrossRef]
- Hohmann, C.; Hohnloser, S.H.; Jacob, J.; Walker, J.; Baldus, S.; Pfister, R. Non-Vitamin K Oral Anticoagulants in Comparison to Phenprocoumon in Geriatric and Non-Geriatric Patients with Non-Valvular Atrial Fibrillation. Thromb. Haemost. 2019, 119, 971–980. [Google Scholar] [CrossRef]
- Hohnloser, S.H.; Basic, E.; Hohmann, C.; Nabauer, M. Effectiveness and Safety of Non-Vitamin K Oral Anticoagulants in Comparison to Phenprocoumon: Data from 61,000 Patients with Atrial Fibrillation. Thromb. Haemost. 2018, 118, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Behr, S.; Andersohn, F.; Garbe, E. Risk of intracerebral hemorrhage associated with phenprocoumon exposure: A nested case-control study in a large population-based German database. Pharmacoepidemiol. Drug Saf. 2010, 19, 722–730. [Google Scholar] [CrossRef]
- Rizos, T.; Jenetzky, E.; Herweh, C.; Hug, A.; Hacke, W.; Steiner, T.; Veltkamp, R. Point-of-care reversal treatment in phenprocoumon-related intracerebral hemorrhage. Ann. Neurol. 2010, 67, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.H.; Gobel, S.; Keller, K.; Coldewey, M.; Ullmann, A.; Lamparter, H.; Junger, C.; Al-Bayati, Z.; Baer, C.; Walter, U.; et al. Quality of oral anticoagulation with phenprocoumon in regular medical care and its potential for improvement in a telemedicine-based coagulation service—Results from the prospective, multi-center, observational cohort study thrombEVAL. BMC Med. 2015, 13, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felli, A.; Zeidler, P.; Jilma, B.; Opfermann, P.; Holaubek, C.; Zimpfer, D.; Wadowski, P.P.; Steinlechner, B. Different Heparin Contents in Prothrombin Complex Concentrates May Impair Blood Clotting in Outpatients with Ventricular Assist Devices Receiving Phenprocoumon. J. Cardiothorac. Vasc. Anesth. 2016, 30, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Yasaka, M.; Sakata, T.; Minematsu, K.; Naritomi, H. Correction of INR by prothrombin complex concentrate and vitamin K in patients with warfarin related hemorrhagic complication. Thromb. Res. 2002, 108, 25–30. [Google Scholar] [CrossRef]
- Sin, J.H.; Berger, K.; Lesch, C.A. Four-factor prothrombin complex concentrate for life-threatening bleeds or emergent surgery: A retrospective evaluation. J. Crit. Care 2016, 36, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Schulman, S.; Dowlatshahi, D.; Holbrook, A.M.; Simpson, C.S.; Shepherd, L.E.; Wells, P.S.; Giulivi, A.; Gomes, T.; Mamdani, M.; et al. Direct Oral Anticoagulant- or Warfarin-Related Major Bleeding: Characteristics, Reversal Strategies, and Outcomes from a Multicenter Observational Study. Chest 2017, 152, 81–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulman, S.; Kearon, C.; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef]



| Variable | Total (n = 115) | Major Bleeding (n = 82) | Urgent Surgery (n = 33) |
|---|---|---|---|
| Male sex, n (%) | 78 (67.8%) | 53 (64.6%) | 25 (75.8%) |
| Age (year) 1 | 78 (72–84) | 79 (73–85) | 76 (66–82) |
| BMI (kg/m2) 1 | 26.3 (23.5–29.2) | 26.3 (23.9–29.6) | 25.6 (22.4–29.2) |
| Dementia (%) | 3 (2.6%) | 1 (1.2%) | 2 (6.1%) |
| Stroke/TIA (%) | 26 (22.4%) | 20 (24.4%) | 6 (18.2%) |
| Cockcroft–Gault formula (mL/min) 1 | 66 (41–92) | 59 (42–92) | 68 (33–97) |
| INR 1 | 2.5 (2.8–3.7) | 2.7 (1.6–4.0) | 2.3 (1.9–2.9) |
| Known liver disease | 5 (4.3%) | 4 (4.9%) | 1 (3.0%) |
| Indication for anticoagulation | |||
| Non-valvular atrial fibrillation (%) | 88 (76.5%) | 65 (79.3%) | 23 (69.7%) |
| CHADS-Vasc Score 1 | 5 (4–6) | 5 (4–6) | 5 (3.5–6.5) |
| HASBLED-Score 1 | 3 (2–3) | 3 (2–3) | 3 (2–3.25) |
| venous thromboembolism (%) | 8 (7.0%) | 7 (8.5%) | 1 (3.0%) |
| Artificial heart valve (%) | 8 (7.0%) | 6 (7.3%) | 2 (6.1%) |
| Vascular risk factors | |||
| Hypertension (%) | 92 (80%) | 69 (84.1%) | 23 (69.7%) |
| Diabetes mellitus (%) | 35 (30.4%) | 24 (29.3%) | 11 (33.3%) |
| Hyperlipidemia (%) | 43 (37.4%) | 32 (39.0%) | 11 (33.3%) |
| Smoking (%) | 15 (13.0%) | 10 (12.2%) | 5 (15.2%) |
| Type of bleeding2 | |||
| Intracranial/intraspinal, n (%) | - | 53 (64.6%) | - |
| GI bleeding, n (%) | - | 17 (20.7%) | - |
| Other locations, n (%) | - | 13 (15.7%) | - |
| Type of surgery2 | |||
| Trauma, n (%) | - | - | 7 (21.2%) |
| Acute abdomen, n (%) | - | - | 11 (33.3%) |
| Other surgery, n (%) | - | - | 15 (45.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindhoff-Last, E.; Birschmann, I.; Bidenharn, A.J.; Kuhn, J.; Lindau, S.; Konstantinides, S.; Grottke, O.; Nowak-Göttl, U.; Lucks, J.; Zydek, B.; et al. Pharmacokinetics of Phenprocoumon in Emergency Situations–Results of the Prospective Observational RADOA-Registry (Reversal Agent Use in Patients Treated with Direct Oral Anticoagulants or Vitamin K Antagonists Registry). Pharmaceuticals 2022, 15, 1437. https://0-doi-org.brum.beds.ac.uk/10.3390/ph15111437
Lindhoff-Last E, Birschmann I, Bidenharn AJ, Kuhn J, Lindau S, Konstantinides S, Grottke O, Nowak-Göttl U, Lucks J, Zydek B, et al. Pharmacokinetics of Phenprocoumon in Emergency Situations–Results of the Prospective Observational RADOA-Registry (Reversal Agent Use in Patients Treated with Direct Oral Anticoagulants or Vitamin K Antagonists Registry). Pharmaceuticals. 2022; 15(11):1437. https://0-doi-org.brum.beds.ac.uk/10.3390/ph15111437
Chicago/Turabian StyleLindhoff-Last, Edelgard, Ingvild Birschmann, Antonia J. Bidenharn, Joachim Kuhn, Simone Lindau, Stavros Konstantinides, Oliver Grottke, Ulrike Nowak-Göttl, Jessica Lucks, Barbara Zydek, and et al. 2022. "Pharmacokinetics of Phenprocoumon in Emergency Situations–Results of the Prospective Observational RADOA-Registry (Reversal Agent Use in Patients Treated with Direct Oral Anticoagulants or Vitamin K Antagonists Registry)" Pharmaceuticals 15, no. 11: 1437. https://0-doi-org.brum.beds.ac.uk/10.3390/ph15111437