Dendrimers Show Promise for siRNA and microRNA Therapeutics
Abstract
:1. Introduction
2. Dendrimers
2.1. Polyamidoamine Dendrimers (PAMAMs)
2.2. Polypropylenimine Dendrimers (PPIs)
2.3. Carbosilane Dendrimers (CBS)
2.4. Polylysine Dendrimers (PLL Dendrimers)
2.5. Phosphorus-Containing Dendrimers
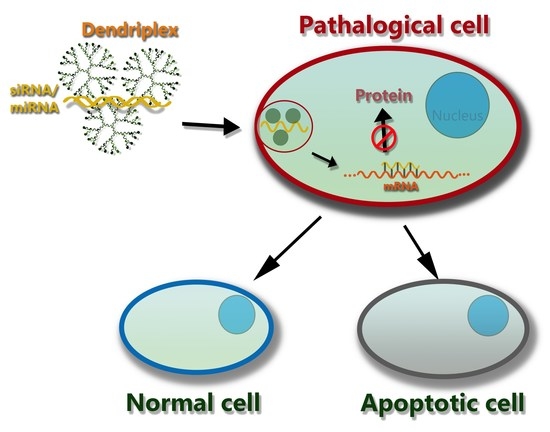
3. The Mechanism of Action of Dendriplexes in a Cell
4. PAMAM + siRNAs Dendriplexes
4.1. PAMAM Surface Decoration with Amino Acids and Peptides
4.2. Surface Decoration with Oligosaccharide
4.3. Core Modification
4.4. Amphiphilic Lipid-Like Dendrons
5. Non-PAMAM + siRNAs Dendriplexes
6. Dendriplexes with microRNA Mimics and Antagonists
- Similar to siRNA, the synthetic mimics of microRNAs (miRs) or their precursors can be introduced into the cell with subsequent target gene silencing triggered by RNA interference.
- Otherwise “malignant” endogenous microRNAs in the cells can be arrested by synthetic oligonucleotides called microRNA antagonists (antimiRs). These oligonucleotides form strong duplexes with microRNAs and block their activity [10].
7. Combined Effect of siRNA and microRNA with Therapeutic Drugs
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TNA | therapeutic nucleic acid |
| RNAi | RNA interference |
| PAMAM | polyamidoamine |
| EDA | ethylenediamine |
| TEA | triethanolamine |
| PPI | polypropylenimine |
| PLL | poly-l-lysine |
| CBS | carbosilane dendrimer |
| Gn | dendrimer of nth generation |
| siRNA | small interfering RNA |
| dsiRNA | Dicer substrate siRNA (siRNA precursor) |
| miR | synthetic mimics of microRNA |
| antimiR | microRNA antagonist |
| PEG | polyethylene glycol |
| PBMC | peripheral blood mononuclear cell |
| CPP | cell-penetrating peptide |
| FA | folic acid |
References
- Kole, R.; Krainer, A.R.; Altman, S. RNA therapeutics: Beyond RNA interference and antisense oligonucleotides. Nat. Rev. Drug Discov. 2012, 11, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Titze-de-Almeida, R.; David, C.; Titze-de-Almeida, S.S. The Race of 10 Synthetic RNAi-Based Drugs to the Pharmaceutical Market. Pharm. Res. 2017, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Borna, H.; Imani, S.; Iman, M.; Azimzadeh Jamalkandi, S. Therapeutic face of RNAi: In vivo challenges. Expert Opin. Biol. Ther. 2015, 15, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Dufès, C.; Uchegbu, I.F.; Schätzlein, A.G. Dendrimers in gene delivery. Adv. Drug Deliv. Rev. 2005, 57, 2177–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niven, R.; Pearlman, R.; Wedeking, T.; Mackeigan, J.; Noker, P.; Simpson-Herren, L.; Smith, J.G. Biodistribution of radiolabeled lipid-DNA complexes and DNA in mice. J. Pharm. Sci. 1998, 87, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Sen, G.L.; Blau, H.M. A brief history of RNAi: The silence of the genes. Faseb J. 2006, 20, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Novina, C.D.; Sharp, P.A. The RNAi revolution. Nature 2004, 430, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.A. RNA interference—2001. Genes Dev. 2001, 15, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Rossi, J.J. Strategies for silencing human disease using RNA interference. Nat. Rev. Genet. 2007, 8, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.K.W.; Chow, M.Y.T.; Zhang, Y.; Leung, S.W.S. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol. Ther. Acids 2015, 4, e252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Yang, B.B. Friend or foe: The role of microRNA in chemotherapy resistance. Acta Pharmacol. Sin. 2013, 34, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Dong, C.; Ji, C. MicroRNA and drug resistance. Cancer Gene Ther. 2010, 17, 523–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daka, A.; Peer, D. RNAi-based nanomedicines for targeted personalized therapy. Adv. Drug Deliv. Rev. 2012, 64, 1508–1521. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, G.; Ozpolat, B.; Coleman, R.L.; Sood, A.K.; Lopez-Berestein, G. Preclinical and clinical development of siRNA-based therapeutics. Adv. Drug Deliv. Rev. 2015, 87, 108–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelnar, K.; Bader, A.G. A qRT-PCR method for determining the biodistribution profi le of a miR-34a mimic. Methods Mol. Biol. 2015, 1317, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Querfeld, C.; Pacheco, T.; Foss, F.M.; Halwani, A.S.; Porcu, P.; Seto, A.G.; Ruckman, J.; Landry, M.L.; Jackson, A.L.; Pestano, L.A.; et al. Preliminary Results of a Phase 1 Trial Evaluating MRG-106, a Synthetic microRNA Antagonist (LNA antimiR) of microRNA-155, in Patients with CTCL. Blood 2016, 128, 1829. [Google Scholar]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, L.; Lai, M.; Chiuppesi, F.; Ceccherini-Nelli, L.; Pistello, M. Viral vectors: A look back and ahead on gene transfer technology. New Microbiol. 2013, 36, 1–22. [Google Scholar] [PubMed]
- Ylä-Herttuala, S. Endgame: Glybera Finally Recommended for Approval as the First Gene Therapy Drug in the European Union. Mol. Ther. 2012, 20, 1831–1832. [Google Scholar] [CrossRef] [PubMed]
- Touchot, N.; Flume, M. Early Insights from Commercialization of Gene Therapies in Europe. Genes 2017, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, Y.; Peng, H.; Chen, Y.; Zhu, P.; Huang, Y. Recent progress in microRNA delivery for cancer therapy by non-viral synthetic vectors. Adv. Drug Deliv. Rev. 2015, 81, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Kanasty, R.; Dorkin, J.R.; Vegas, A.; Anderson, D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013, 12, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Dahlman, J.E.; Kauffman, K.J.; Langer, R.; Anderson, D.G. Nanotechnology for In vivo Targeted siRNA Delivery. Adv. Genet. 2014, 88, 37–69. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Caminade, A.-M.; Ouali, A.; Laurent, R.; Turrin, C.-O.; Majoral, J.-P. The dendritic effect illustrated with phosphorus dendrimers. Chem. Soc. Rev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Haensler, J.; Szoka, F.C.J. Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjugate Chem. 1993, 4, 372–379. [Google Scholar] [CrossRef]
- Bauer, B.J.; Amis, E.J. Characterization of Dendritically Branched Polymers by Small Angle Neutron Scattering (SANS), Small Angle X-Ray Scattering (SAXS) and Transmission Electron Microscopy (TEM). In Dendrimers and Other Dendritic Polymers; John Wiley & Sons, Ltd.: Chichester, UK, 2001; pp. 255–284. ISBN 9780470845820. [Google Scholar]
- Mintzer, M.A.; Grinstaff, M.W. Biomedical applications of dendrimers: A tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Aval, S.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.; Joo, S.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Bioconjug. Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Dendrimers: Towards Catalytic, Material and Biomedical Uses; Caminade, A.-M.; Turrin, C.-O.; Laurent, R.; Ouali, A.; Delavaux-Nicot, B. (Eds.) John Wiley & Sons Ltd.: Chichester, UK, 2011; ISBN 9781119976530. [Google Scholar]
- Dendrimers in Biomedical Applications; Klajnert, B.; Peng, L.; Cena, V. (Eds.) Royal Society of Chemistry: Cambridge, UK, 2013; ISBN 978-1-84973-611-4. [Google Scholar]
- Caminade, A.M.; Turrin, C.O.; Majoral, J.P. Biological properties of phosphorus dendrimers. New J. Chem. 2010. [Google Scholar]
- Khan, O.F.; Zaia, E.W.; Yin, H.; Bogorad, R.L.; Pelet, J.M.; Webber, M.J.; Zhuang, I.; Dahlman, J.E.; Langer, R.; Anderson, D.G. Ionizable amphiphilic dendrimer-based nanomaterials with alkyl-chain-substituted amines for tunable sirna delivery to the liver endothelium in vivo. Angew. Chem. Int. Ed. 2014, 53, 14397–14401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Neff, C.P.; Liu, X.; Zhang, J.; Li, H.; Smith, D.D.; Swiderski, P.; Aboellail, T.; Huang, Y.; Du, Q.; et al. Systemic administration of combinatorial dsiRNAs via nanoparticles efficiently suppresses HIV-1 infection in humanized mice. Mol. Ther. 2011, 19, 2228–2238. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, A.C.; Rivilla, I.; Perez-Martinez, F.C.; Monteagudo, S.; Ocana, V.; Guerra, J.; Garcia-Martinez, J.C.; Merino, S.; Sanchez-Verdu, P.; Cena, V.; et al. Efficient, non-toxic hybrid PPV-PAMAM dendrimer as a gene carrier for neuronal cells. Biomacromolecules 2011, 12, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Christensen, J.B.; Boas, U. Dendrimers, Dendrons, and Dendritic Polymers, Discovery, Applications, and the Future; Cambridge University Press: Cambridge, UK, 2012; 412p. [Google Scholar]
- Liu, X.; Li, G.; Su, Z.; Jiang, Z.; Chen, L.; Wang, J.; Yu, S.; Liu, Z. Poly(amido amine) is an ideal carrier of miR-7 for enhancing gene silencing effects on the EGFR pathway in U251 glioma cells. Oncol. Rep. 2013, 29, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, C.; Zhou, J.; Chen, C.; Qu, F.; Rossi, J.J.; Rocchi, P.; Peng, L. Promoting siRNA delivery via enhanced cellular uptake using an arginine-decorated amphiphilic dendrimer. Nanoscale 2015, 7, 3867–3875. [Google Scholar] [CrossRef] [PubMed]
- Dehshahri, A.; Sadeghpour, H. Surface decorations of poly(amidoamine) dendrimer by various pendant moieties for improved delivery of nucleic acid materials. Colloids Surf. B Biointerfaces 2015, 132, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Van Duijvenbode, R.C.; Borkovec, M.; Koper, G.J.M. Acid-base properties of poly(propylene imine)dendrimers. Polymer 1998, 39, 2657–2664. [Google Scholar] [CrossRef]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Santhakumaran, L.M. Enhanced cellular uptake of a triplex-forming oligonucleotide by nanoparticle formation in the presence of polypropylenimine dendrimers. Nucleic Acids Res. 2004, 32, 2102–2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinselmeyer, B.H.; Mackay, S.P.; Schatzlein, A.G.; Uchegbu, I.F. The lower-generation polypropylenimine dendrimers are effective gene-transfer agents. Pharm. Res. 2002, 19, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, J.F.; Ortega, P.; Chonco, L.; Eritja, R.; Samaniego, R.; Müllner, M.; De Jesus, E.; De La Mata, F.J.; Flores, J.C.; Gomez, R.; et al. Water-soluble carbosilane dendrimers: Synthesis biocompatibility and complexation with oligonucleotides; evaluation for medical applications. Chem. A Eur. J. 2007, 13, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Ortega, P.; Bermejo, J.F.; Chonco, L.; De Jesus, E.; De La Mata, F.J.; Fernández, G.; Flores, J.C.; Gómez, R.; Serramía, M.J.; Angeles Muñoz-Fernandez, M. Novel water-soluble carbosilane dendrimers: Synthesis and biocompatibility. Eur. J. Inorg. Chem. 2006, 1388–1396. [Google Scholar] [CrossRef]
- Pedziwiatr-Werbicka, E.; Fuentes, E.; Dzmitruk, V.; Sánchez-Nieves, J.; Sudas, M.; Drozd, E.; Shakhbazau, A.; Shcharbin, D.; de la Mata, F.J.; Gomez-Ramirez, R.; et al. Novel “SiC” carbosilane dendrimers as carriers for anti-HIV nucleic acids: Studies on complexation and interaction with blood cells. Colloids Surf. B Biointerfaces 2013, 109, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Ionov, M.; Lazniewska, J.; Dzmitruk, V.; Halets, I.; Loznikova, S.; Novopashina, D.; Apartsin, E.; Krasheninina, O.; Venyaminova, A.; Milowska, K.; et al. Anticancer siRNA cocktails as a novel tool to treat cancer cells. Part (A). Mechanisms of interaction. Int. J. Pharm. 2015, 485, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Dzmitruk, V.; Szulc, A.; Shcharbin, D.; Janaszewska, A.; Shcharbina, N.; Lazniewska, J.; Novopashina, D.; Buyanova, M.; Ionov, M.; Klajnert-Maculewicz, B.; et al. Anticancer siRNA cocktails as a novel tool to treat cancer cells. Part (B). Efficiency of pharmacological action. Int. J. Pharm. 2015, 485, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.L.; Clemente, M.I.; Weber, N.D.; Sanchez, J.; Ortega, P.; de la Mata, F.J.; Gomez, R.; Garcia, D.; Lopez-Fernandez, L.A.; Munoz-Fernandez, M.A. Carbosilane dendrimers to transfect human astrocytes with small interfering RNA targeting human immunodeficiency virus. BioDrugs 2010, 24, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Posadas, I.; López-Hernández, B.; Clemente, M.I.; Jiménez, J.L.; Ortega, P.; De La Mata, J.; Gómez, R.; Muñoz-Fernández, M.A.; Ceña, V. Highly efficient transfection of rat cortical neurons using carbosilane dendrimers unveils a neuroprotective Role for HIF-1α in early chemical hypoxia-mediated neurotoxicity. Pharm. Res. 2009, 26, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Serramía, M.J.; Álvarez, S.; Fuentes-Paniagua, E.; Clemente, M.I.; Sánchez-Nieves, J.; Gómez, R.; De La Mata, J.; Muñoz-Fernández, M.Á. In vivo delivery of siRNA to the brain by carbosilane dendrimer. J. Control. Release 2015, 200, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Kadlecova, Z.; Rajendra, Y.; Matasci, M.; Baldi, L.; Hacker, D.L.; Wurm, F.M.; Klok, H.A. DNA delivery with hyperbranched polylysine: A comparative study with linear and dendritic polylysine. J. Control. Release 2013, 169, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, M.; Okuda, T.; Wada, A.; Hirayama, T.; Niidome, T.; Aoyagi, H. In Vitro Gene Transfection Using Dendritic Poly(l-lysine). Bioconjug. Chem. 2002, 13, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Kidoaki, S.; Ohsaki, M.; Koyama, Y.; Yoshikawa, K.; Niidome, T.; Aoyagi, H. Time-dependent complex formation of dendritic poly(l-lysine) with plasmid DNA and correlation with in vitro transfection efficiencies. Org. Biomol. Chem. 2003, 1, 1270–1273. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Sugiyama, A.; Niidome, T.; Aoyagi, H. Characters of dendritic poly(l-lysine) analogues with the terminal lysines replaced with arginines and histidines as gene carriers in vitro. Biomaterials 2004, 25, 537–544. [Google Scholar] [CrossRef]
- Yamagata, M.; Kawano, T.; Shiba, K.; Mori, T.; Katayama, Y.; Niidome, T. Structural advantage of dendritic poly(l-lysine) for gene delivery into cells. Bioorg. Med. Chem. 2007, 15, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.-M.; Majoral, J.-P. Positively charged phosphorus dendrimers. An overview of their properties. New J. Chem. 2013, 37, 3358–3373. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Majoral, J.-P. Water-soluble phosphorus-containing dendrimers. Prog. Polym. Sci. 2005, 30, 491–505. [Google Scholar] [CrossRef]
- Majoral, J.-P.; Caminade, A.-M.; Laurent, R.; Sutra, P. Phosphorus-containing dendrimers: From material science to biology. Heteroat. Chem. 2002, 13, 474–485. [Google Scholar] [CrossRef]
- Mu, R.; Laschober, C.; Szymanski, W.W. Determination of Molecular Weight, Particle Size, and Density of High Number Generation PAMAM Dendrimers Using MALDI-TOF-MS and nES-GEMMA. Macromolecules 2007, 40, 5599–5605. [Google Scholar] [CrossRef]
- Loup, C.; Zanta, M.; Caminade, A. Preparation of Water-Soluble Cationic Phosphorus-Containing Dendrimers. Chem. Eur. J. 1999, 5, 3644–3650. [Google Scholar] [CrossRef]
- Padié, C.; Maszewska, M.; Majchrzak, K.; Nawrot, B.; Caminade, A.-M.; Majoral, J.-P. Polycationic phosphorus dendrimers: Synthesis, characterization, study of cytotoxicity, complexation of DNA, and transfection experiments. New J. Chem. 2009, 33, 318–326. [Google Scholar] [CrossRef]
- D’Emanuele, A.; Attwood, D. Dendrimer-drug interactions. Adv. Drug Deliv. Rev. 2005, 57, 2147–2162. [Google Scholar] [CrossRef] [PubMed]
- Shcharbin, D.; Pedziwiatr, E.; Bryszewska, M. How to study dendriplexes I: Characterization. J. Control. Release 2009, 135, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Shcharbin, D.; Pedziwiatr, E.; Blasiak, J.; Bryszewska, M. How to study dendriplexes II: Transfection and cytotoxicity. J. Control. Release 2010, 141, 110–127. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; van Dongen, M.A.; Han, Y.; Yu, M.; Li, Y.; Liu, G.; Banaszak Holl, M.M.; Qi, R. The role of caveolin-1 and syndecan-4 in the internalization of PEGylated PAMAM dendrimer polyplexes into myoblast and hepatic cells. Eur. J. Pharm. Biopharm. 2014, 88, 658–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A. V Endocytosis of nanomedicines. J. Control. Release 2010, 145, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Hatakeyama, H.; Sato, Y.; Akita, H.; Takayama, K.; Kobayashi, S.; Futaki, S.; Harashima, H. Endosomal escape and the knockdown efficiency of liposomal-siRNA by the fusogenic peptide shGALA. Biomaterials 2011, 32, 5733–5742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rejman, J.; Bragonzi, A.; Conese, M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol. Ther. 2005, 12, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Szoka, F.C. Nucleic acid delivery: The missing pieces of the puzzle? Acc. Chem. Res. 2012, 45, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.; Dougherty, C.A.; Xue, Y.; Al-Hashimi, H.M.; Banaszak Holl, M.M. Rapid Exchange Between Free and Bound States in RNA–Dendrimer Polyplexes: Implications on the Mechanism of Delivery and Release. Biomacromolecules 2016, 17, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, M.; Drzewinska, J.; Dzmitruk, V.; Shcharbin, D.; Klajnert, B.; Appelhans, D.; Bryszewska, M. Stability of Dendriplexes Formed by Anti-HIV Genetic Material and Poly(propylene imine) Dendrimers in the Presence of Glucosaminoglycans. J. Phys. Chem. B 2012, 116, 14525–14532. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N.K. Dendrimer toxicity: Let’s meet the challenge. Int. J. Pharm. 2010, 394, 122–142. [Google Scholar] [CrossRef] [PubMed]
- Shcharbin, D.; Ionov, M.; Abashkin, V.; Loznikova, S.; Dzmitruk, V.; Shcharbina, N.; Matusevich, L.; Milowska, K.; Gałecki, K.; Wysocki, S.; et al. Nanoparticle corona for proteins: Mechanisms of interaction between dendrimers and proteins. Colloids Surf. B Biointerfaces 2015, 134, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Akesson, A.; Cardenas, M.; Elia, G.; Monopoli, M.P.; Dawson, K.A. The protein corona of dendrimers: PAMAM binds and activates complement proteins in human plasma in a generation dependent manner. RSC Adv. 2012, 2, 11245–11248. [Google Scholar] [CrossRef]
- Shcharbin, D.; Janaszewska, A.; Klajnert-Maculewicz, B.; Ziemba, B.; Dzmitruk, V.; Halets, I.; Loznikova, S.; Shcharbina, N.; Milowska, K.; Ionov, M.; et al. How to study dendrimers and dendriplexes III. Biodistribution, pharmacokinetics and toxicity in vivo. J. Control. Release 2014, 181, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Russ, V.; Gnther, M.; Halama, A.; Ogris, M.; Wagner, E. Oligoethylenimine-grafted polypropylenimine dendrimers as degradable and biocompatible synthetic vectors for gene delivery. J. Control. Release 2008, 132, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Dennig, J.; Duncan, E. Gene transfer into eukaryotic cells using activated polyamidoamine dendrimers. J. Biotechnol. 2002, 90, 339–347. [Google Scholar] [CrossRef]
- Al-Jamal, K.T.; Ramaswamy, C.; Florence, A.T. Supramolecular structures from dendrons and dendrimers. Adv. Drug Deliv. Rev. 2005, 57, 2238–2270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, J.; Xiao, C.; Li, M.; Tian, H.; Chen, X. Cationic dendron-bearing lipids: Investigating structure-activity relationships for small interfering RNA delivery. Biomacromolecules 2013, 14, 4289–4300. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, X.; Rocchi, P.; Qu, F.; Iovanna, J.L.; Peng, L. Arginine-terminated generation 4 PAMAM dendrimer as an effective nanovector for functional siRNA delivery in vitro and in vivo. Bioconjug. Chem. 2014, 25, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, C.; Chen, C.; Bentobji, M.; Cheillan, F.A.; Piana, J.T.; Qu, F.; Rocchi, P.; Peng, L. Targeted delivery of Dicer-substrate siRNAs using a dual targeting peptide decorated dendrimer delivery system. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Liu, X.; Bolcato-Bellemin, A.-L.; Wang, Y.; Liu, C.; Erbacher, P.; Qu, F.; Rocchi, P.; Behr, J.-P.; Peng, L. An amphiphilic dendrimer for effective delivery of small interfering RNA and gene silencing in vitro and in vivo. Angew. Chem. Int. Ed. Engl. 2012, 51, 8478–8484. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, J.; Yu, T.; Chen, C.; Cheng, Q.; Sengupta, K.; Huang, Y.; Li, H.; Liu, C.; Wang, Y.; et al. Adaptive amphiphilic dendrimer-based nanoassemblies as robust and versatile siRNA delivery systems. Angew. Chem. Int. Ed. 2014, 53, 11822–11827. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Zhang, Y.; Li, L.; Cheng, Y. Efficient delivery of small interfering RNA into cancer cells using dodecylated dendrimers. J. Mater. Chem. B 2015, 3, 8197–8202. [Google Scholar] [CrossRef]
- Patil, M.L.; Zhang, M.; Betigeri, S.; Taratula, O.; He, H.; Minko, T. Surface-modified and internally cationic polyamidoamine dendrimers for efficient siRNA delivery. Bioconjug. Chem. 2008, 19, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.L.; Zhang, M.; Taratula, O.; Garbuzenko, O.B.; He, H.; Minko, T. Internally cationic polyamidoamine PAMAM-OH dendrimers for siRNA delivery: Effect of the degree of Quaternization and cancer targeting. Biomacromolecules 2009, 10, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.L.; Zhang, M.; Minko, T. Multifunctional triblock nanocarrier (PAMAM-PEG-PLL) for the efficient intracellular siRNA delivery and gene silencing. ACS Nano 2011, 5, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; DeLong, R.; Fisher, M.H.; Juliano, R.L. Tat-Conjugated PAMAM Dendrimers as Delivery Agents for Antisense and siRNA Oligonucleotides. Pharm. Res. 2005, 22, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Anno, T.; Higashi, T.; Hayashi, Y.; Motoyama, K.; Jono, H.; Ando, Y.; Arima, H. Potential use of glucuronylglucosyl-β-cyclodextrin/dendrimer conjugate (G2) as a siRNA carrier for the treatment of familial amyloidotic polyneuropathy. J. Drug Target. 2014, 22, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, K.; Mitsuyasu, R.; Akao, C.; Abu Hashim, I.I.; Sato, N.; Tanaka, T.; Higashi, T.; Arima, H. Potential Use of Thioalkylated Mannose-Modified Dendrimer (G3)/α-Cyclodextrin Conjugate as an NF-κB siRNA Carrier for the Treatment of Fulminant Hepatitis. Mol. Pharm. 2015, 12, 3129–3136. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, H.-C.; Zhang, L.-M.; Deng, J.-J.; Xie, X.-Y.; Lin, D.-Z.; Ren, M.; Yang, C.; Yan, L. Cationic star-shaped polymer as an siRNA carrier for reducing MMP-9 expression in skin fibroblast cells and promoting wound healing in diabetic rats. Int. J. Nanomed. 2014, 9, 3377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Gu, C.; Cabigas, E.B.; Pendergrass, K.D.; Brown, M.E.; Luo, Y.; Davis, M.E. Functionalized dendrimer-based delivery of angiotensin type 1 receptor siRNA for preserving cardiac function following infarction. Biomaterials 2013, 34, 3729–3736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Carriõn, M.D.; Pérez-Martínez, F.C.; Merino, S.; Sánchez-Verdã, P.; Martínez-Hernández, J.; Luján, R.; Ceña, V. Dendrimer-mediated siRNA delivery knocks down Beclin 1 and potentiates NMDA-mediated toxicity in rat cortical neurons. J. Neurochem. 2012, 120, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Finlay, J.; Roberts, C.M.; Lowe, G.; Loeza, J.; Rossi, J.J.; Glackin, C.A. RNA-based TWIST1 inhibition via dendrimer complex to reduce breast cancer cell metastasis. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Harada-Shiba, M.; Suzuki, A.; Gokuden, R.; Kurihara, R.; Sugao, Y.; Mori, T.; Katayama, Y.; Niidome, T. In vivo siRNA delivery with dendritic poly(l-lysine) for the treatment of hypercholesterolemia. Mol. Biosyst. 2009, 5, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Kurihara, R.; Tsuchida, A.; Hasegawa, M.; Nagashima, T.; Mori, T.; Niidome, T.; Katayama, Y.; Okitsu, O. Efficient delivery of siRNA using dendritic poly(l-lysine) for loss-of-function analysis. J. Control. Release 2008, 126, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Perisé-Barrios, A.J.; Jiménez, J.L.; D’Omínguez-Soto, A.; De La Mata, F.J.; Corbí, A.L.; Gomez, R.; Muñoz-Fernandez, M.Á. Carbosilane dendrimers as gene delivery agents for the treatment of HIV infection. J. Control. Release 2014, 184, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Briz, V.; Serramía, M.J.; Madrid, R.; Hameau, A.; Caminade, A.-M.; Majoral, J.P.; Muñoz-Fernández, M.A. Validation of a generation 4 phosphorus-containing polycationic dendrimer for gene delivery against HIV-1. Curr. Med. Chem. 2012, 19, 5044–5051. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo, T.; Clemente, M.I.; Chonco, L.; Weber, N.D.; Díaz, L.; Serramía, M.J.; Gras, R.; Ortega, P.; De La Mata, F.J.; Gómez, R.; et al. Gene therapy in HIV-infected cells to decrease viral impact by using an alternative delivery method. ChemMedChem 2010, 5, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Weber, N.; Ortega, P.; Clemente, M.I.; Shcharbin, D.; Bryszewska, M.; de la Mata, F.J.; Gómez, R.; Muñoz-Fernández, M.A. Characterization of carbosilane dendrimers as effective carriers of siRNA to HIV-infected lymphocytes. J. Control. Release 2008, 132, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Taratula, O.; Garbuzenko, O.B.; Kirkpatrick, P.; Pandya, I.; Savla, R.; Pozharov, V.P.; He, H.; Minko, T. Surface-engineered targeted PPI dendrimer for efficient intracellular and intratumoral siRNA delivery. J. Control. Release 2009, 140, 284–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziemba, B.; Halets, I.; Shcharbin, D.; Appelhans, D.; Voit, B.; Pieszynski, I.; Bryszewska, M.; Klajnert, B. Influence of fourth generation poly(propyleneimine) dendrimers on blood cells. J. Biomed. Mater. Res. Part A 2012, 100A, 2870–2880. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Posocco, P.; Liu, X.; Cheng, Q.; Laurini, E.; Zhou, J.; Liu, C.; Wang, Y.; Tang, J.; Col, V.D.; et al. Mastering Dendrimer Self-Assembly for Efficient siRNA Delivery: From Conceptual Design to In Vivo Efficient Gene Silencing. Small 2016, 3667–3676. [Google Scholar] [CrossRef] [PubMed]
- Shcharbin, D.; Pedziwiatr, E.; Nowacka, O.; Kumar, M.; Zaborski, M.; Ortega, P.; Javier de la Mata, F.; Gómez, R.; Muñoz-Fernandez, M.A.; Bryszewska, M. Carbosilane dendrimers NN8 and NN16 form a stable complex with siGAG1. Colloids Surf. B Biointerfaces 2011, 83, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Pedziwiatr-Werbicka, E.; Shcharbin, D.; Maly, J.; Maly, M.; Zaborski, M.; Gabara, B.; Ortega, P.; Javier De La Mata, F.; Gómez, R.; Angeles Muñoz-Fernandez, M.; et al. Carbosilane dendrimers are a non-viral delivery system for antisense oligonucleotides: Characterization of dendriplexes. J. Biomed. Nanotechnol. 2012, 8, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21 ± nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.L.; McCray, P.B. Current prospects for RNA interference-based therapies. Nat. Rev. Genet. 2011, 12, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.P.; Lau, N.C.; Garrett-Engele, P.; Grimson, A.; Schelter, J.M.; Castle, J.; Bartel, D.P.; Linsley, P.S.; Johnson, J.M. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005, 433, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Yingchun Tong, J.A.S. Switching from Repression to Activation: MicroRNAs Can Up-Regulate Translation. Science 2007, 318, 1931–1934. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNA Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Ali, S.; Sethi, S.; Sarkar, F.H. MicroRNAs in personalized cancer therapy. Clin. Genet. 2014, 86, 68–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budak, H.; Bulut, R.; Kantar, M.; Alptekin, B. MicroRNA nomenclature and the need for a revised naming prescription. Brief. Funct. Genom. 2016, 15, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Desvignes, T.; Batzel, P.; Berezikov, E.; Eilbeck, K.; Eppig, J.T.; McAndrews, M.S.; Singer, A.; Postlethwait, J.H. MiRNA Nomenclature: A View Incorporating Genetic Origins, Biosynthetic Pathways, and Sequence Variants. Trends Genet. 2015, 31, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.D.; Wu, R.J.; Yin, X.; Zhou, J.; Davis, M.E.; Luo, Y. Dendrimeric bowties featuring hemispheric-selective decoration of ligands for microRNA-based therapy. Biomacromolecules 2013, 14, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Nguyen, L.H.; Miller, J.B.; Yan, Y.; Kos, P.; Xiong, H.; Li, L.; Hao, J.; Minnig, J.T.; Zhu, H.; et al. Modular degradable dendrimers enable small RNAs to extend survival in an aggressive liver cancer model. Proc. Natl. Acad. Sci. USA 2016, 113, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Tiram, G.; Segal, E.; Krivitsky, A.; Shreberk-Hassidim, R.; Ferber, S.; Ofek, P.; Udagawa, T.; Edry, L.; Shomron, N.; Roniger, M.; et al. Identification of Dormancy-Associated MicroRNAs for the Design of Osteosarcoma-Targeted Dendritic Polyglycerol Nanopolyplexes. ACS Nano 2016, 10, 2028–2045. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Xing, Z.; Yang, J.; Wang, Y.; Chen, J.; Zhang, Y.; Shi, W.; Li, Q. Chondroitin sulfate-functionalized polyamidoamine-mediated miR-34a delivery for inhibiting the proliferation and migration of pancreatic cancer. RSC Adv. 2016, 6, 70870–70876. [Google Scholar] [CrossRef]
- Campbell, M.A.; Wengel, J. Locked vs. unlocked nucleic acids (LNAvs.UNA): Contrasting structures work towards common therapeutic goals. Chem. Soc. Rev. 2011, 40, 5680–5689. [Google Scholar] [CrossRef] [PubMed]
- Kala, S.; Mak, A.S.C.; Liu, X.; Posocco, P.; Pricl, S.; Peng, L.; Wong, A.S.T. Combination of dendrimer-nanovector-mediated small interfering RNA delivery to target akt with the clinical anticancer drug paclitaxel for effective and potent anticancer activity in treating ovarian cancer. J. Med. Chem. 2014, 57, 2634–2642. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo, S.; Pérez-Martínez, F.C.; Pérez-Carrión, M.D.; Guerra, J.; Merino, S.; Sánchez-Verdú, M.P.; Ceña, V. Inhibition of p42 MAPK using a nonviral vector-delivered siRNA potentiates the anti-tumor effect of metformin in prostate cancer cells. Nanomedicine 2012, 7, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Lv, Q.; Tang, X.J.; Hu, Y.L.; Xu, D.H.; Li, F.Z.; Liang, W.Q.; Gao, J.Q. Overcoming drug resistance of MCF-7/ADR cells by altering intracellular distribution of doxorubicin via MVP knockdown with a novel siRNA polyamidoamine-hyaluronic acid complex. J. Control. Release 2012, 163, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Ihnatsyeu-Kachan, A.; Dzmitruk, V.; Apartsin, E.; Krasheninina, O.; Ionov, M.; Loznikova, S.; Venyaminova, A.; Miłowska, K.; Shcharbin, D.; Mignani, S.; et al. Multi-Target Inhibition of Cancer Cell Growth by siRNA Cocktails and 5-Fluorouracil Using Effective Piperidine-Terminated Phosphorus Dendrimers. Colloids and Interfaces 2017, 1, 6. [Google Scholar] [CrossRef]
- Ren, Y.; Kang, C.-S.; Yuan, X.-B.; Zhou, X.; Xu, P.; Han, L.; Wang, G.X.; Jia, Z.; Zhong, Y.; Yu, S.; et al. Co-delivery of as-miR-21 and 5-FU by poly(amidoamine) dendrimer attenuates human glioma cell growth in vitro. J. Biomater. Sci. Polym. Ed. 2010, 21, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhou, X.; Mei, M.; Yuan, X.-B.; Han, L.; Wang, G.-X.; Jia, Z.-F.; Xu, P.; Pu, P.-Y.; Kang, C.-S. MicroRNA-21 inhibitor sensitizes human glioblastoma cells U251 (PTEN-mutant) and LN229 (PTEN-wild type) to taxol. BMC Cancer 2010, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.; Ren, Y.; Zhou, X.; Yuan, X.-B.; Li, F.; Jiang, L.-H.; Kang, C.-S.; Yao, Z. Suppression of breast cancer cells in vitro by polyamidoamine-dendrimer-mediated 5-fluorouracil chemotherapy combined with antisense micro-RNA 21 gene therapy. J. Appl. Polym. Sci. 2009, 114, 3760–3766. [Google Scholar] [CrossRef]
- Mei, M.; Ren, Y.; Zhou, X.; Yuan, X.; Han, L.; Wang, G.; Jia, Z.; Pu, P.; Kang, C.; Yao, Z. Downregulation of miR-21 enhances chemotherapeutic effect of taxol in breast carcinoma cells. Technol. cancer Res. Treat. 2010, 9, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.-M.; Shi, Z.-D.; Ren, Y.; Liu, C.-Y.; Ji, Y.-R.; Long, L.-X.; Pu, P.; Sheng, J.; Yuan, X.-B.; Kang, C.-S. Synergistic inhibition of human glioma cell line by temozolomide and PAMAM-mediated miR-21i. J. Appl. Polym. Sci. 2013, 127, 570–576. [Google Scholar] [CrossRef]
- Qian, X.; Ren, Y.; Shi, Z.; Long, L.; Pu, P.; Sheng, J.; Yuan, X.; Kang, C. Sequence-dependent synergistic inhibition of human glioma cell lines by combined Temozolomide and miR-21 inhibitor gene therapy. Mol. Pharm. 2012, 9, 2636–2645. [Google Scholar] [CrossRef] [PubMed]
- Shcharbin, D.; Shakhbazau, A.; Bryszewska, M. Poly(amidoamine) dendrimer complexes as a platform for gene delivery. Expert Opin. Drug Deliv. 2013, 10, 1687–1698. [Google Scholar] [CrossRef] [PubMed]
- Mello, C.C.; Conte, D. Revealing the world of RNA interference. Nature 2004, 431, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Doss, C.G.P.; Lee, S.S. Therapeutic miRNA and siRNA: Moving from Bench to Clinic as Next Generation Medicine. Mol. Ther. Nucleic Acids 2017, 8, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Rittenhouse, K.D.; Johnson, T.R.; Vicini, P.; Hirakawa, B.; Kalabat, D.; Yang, A.H.; Huang, W.; Basile, A.S. RTP801 Gene Expression Is Differentially Upregulated in Retinopathy and Is Silenced by PF-04523655, a 19-Mer siRNA Directed Against RTP801Pharmacodynamics of siRNA PF-655 in Retinopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1232–1240. [Google Scholar] [CrossRef] [PubMed]




| Target Protein/Type of Short RNA | Object | Dendrimer/Dendrimer Based Construction | Effect | Ref. |
|---|---|---|---|---|
| PAMAM dendrimers | ||||
| Hsp27/siRNA | Human prostate cancer cells (PC-3) | Arginine-terminated TEA-PAMAM G4 (G4Arg) dendrimer | 80% reduction of Hsp27-mRNA, Hsp27 protein expression dropped by 85% | [82] |
| PC-3 prostate cancer xenografts in nude mice | Hsp27 protein expression decreased by 55% | |||
| Human prostate cancer cells (PC-3) | Complex of TEA-PAMAM G5/siRNA/oligopeptide E16G6RGDK | Hsp27 mRNA reduction by 60%, decrease of Hsp27 protein expression by 85%, reduction of cell viability by 55% | [83] | |
| PC-3 prostate cancer xenografts in nude mice | Hsp27 expression decrease by 70%, 5-fold inhibition of tumor growth | |||
| Human prostate cancer cells (PC-3) | Amphiphilic TEA-PAMAM dendrons G1,2,3, bearing C18 alkyl chain in focal point | Target mRNA decrease of 75%, protein expression decrease of 80%, 2.7-fold increase of apoptotic cells | [84] | |
| PC-3 prostate cancer xenografts in nude mice | Decrease of target mRNA by 50%, decrease of Hsp27 protein expression by 50% | |||
| Human prostate cancer cells (PC-3) | Arginine-decorated TEA-PAMAM Dendron G3, bearing an alkyl chain in the focal point | Decrease of target mRNA by 80% | [39] | |
| Amphiphilic Janus-type PAMAM G2 dendron bearing two alkyl chains in focal point | Decrease of Hsp27 mRNA by 80%, decrease of Hsp27 protein expression by 95% | [85] | ||
| PC-3 prostate cancer xenografts in nude mice | Decrease of hsp27 mRNA by 60%, decrease of Hsp27 protein expression by 75%, 2.5-fold inhibition of tumor growth in vivo | |||
| Cocktail of viral (HIV) Tat and Rev, lymphocytic CD4/TNPO3/dsiRNAs | T-cells and primary human PBMC | TEA-PAMAM G5 dendrimer | Decrease of viral p24 expression by >50% and CD4 expression by 60–75% | [35] |
| HIV-infected humanized Rag2−/−γc−/− mouse model | Decrement of viral load up to 0%, prevent CD4+ T-cell level fall | |||
| Bcl-2 (inhibitor of apoptosis)/siRNA | Human cervical adenocarcinoma cells (HeLa) | Dodecylated PAMAM G4 bearing 23 chains of C12 | Decrease of target mRNA by 90%, protein Bcl-2 expression inhibition by 40% | [86] |
| Human ovarian carcinoma cells (A2780) | QPAMAM-NHAc, internally quaternized and surface-acetylated PAMAM G4 modified with LHRH at the periphery | Inhibition of target mRNA by 85% | [87,88] | |
| Triblock PAMAM-PEG-PLL nanocarrier | Inhibition of target mRNA by up to 80% | [89] | ||
| Alpha-fetoprotein (AFP)/siRNA | C57BL/6 mice, hepatocarcinoma model | EDA-PAMAM G1 substituted by alkyl chains on the periphery | Selective accumulation in hepatocytes, decrease of target protein expression by 50% (C12) and by 90% (C15) | [34] |
| Multiple drug resistance protein 1 (MDR1)/siRNA | MDR1-positive mouse embryonic fibroblast (NIH 3T3) cells | Tat-Conjugated EDA-PAMAM G5 dendrimers | Target protein (MDR1) expression decreased by 35% | [90] |
| Transthyretin—transport protein (TTR)/siRNA | Hepato-carcinoma cells (HepG2) | EDA-PAMAM G2 decorated with glucuronylglucosyl-β-cyclodextrin | Decrease of target mRNA level by 60% | [91] |
| Mice model BALB/c | Decrease of target protein (TTR) expression by 10% | |||
| NF-κB p65- (regulator of inflammatory response)/siRNA | Rat alveolar macrophages (NR8383) | EDA-PAMAM G3- decorated with cyclodextrin and thioalkylated mannose fragments | Decrease of target NF-κB p65 mRNA level by 85% | [92] |
| Mice model C57BL/6 | Reduction of proinflammatory cytokines p65, TNF-α, IL-1β secretion by 75–85% | |||
| MMP-9 (diabetic wound healing regulator)/siRNA | Rat fibroblasts (CRL1213) | PAMAM G3 with β- cyclodextrin core | Decrease of target MMP-9 mRNA level by 68%, decrease of target protein expression by 94% | [93] |
| Sprague Dawley rats with induced diabetes | Enhancement wound healing (52% against 38% in control) | |||
| Cocktail of Bcl-2, Bcl-xL, Mcl-1 (apoptosis inhibitors)/siRNAs | Human cervical adenocarcinoma cells (HeLa), human promyelocytic leukemia cells (HL-60) | PAMAM G3 and G4 | Increased apoptotic cell fraction up to 30–40% | [48,49] |
| Angiotensin II receptor type 1 (AT1R)/siRNA | Cardiomyoblastic cells (H9C2) | EDA-PAMAM G4 dendrons conjugated with PEG-R9peptide | Reduction of protein AT1R expression by 60% | [94] |
| Rats with induced ischemia | 2.5-fold decrease of heart attack risk | |||
| Cofilin-1 (regulator of neuronal death)/siRNA | Rat cerebellar granular neurons (CGNs) | TRANSGEDEN: Polyphenylenevinylene (PPV) core with flexible PAMAM branches | Knockdown of target mRNA by 85%, reduction of protein Ccofilin-1 expression by 80% | [36] |
| Beclin 1 (autophagy regulator)/siRNA | Rat brain rat neurons | Decrease of Beclin 1 mRNA by 90%, knockdown of Beclin 1 protein expression by 80% | [95] | |
| TWIST1 (marker of breast cancer)/siRNA | Breast cancer cells (SUM1315) | YTZ3-15, TEA-PAMAM dendron G3 with two lipid tails in focal point | Decrease of TWIST1 mRNA and protein by 75–95%, reduction of epithelial-mesenchymal transition (EMT)-related (N-cadherin and vimentin) gene mRNA | [96] |
| CD4 (primary HIV receptor)/dsiRNA | Human hematopoietic CD34+ stem cells | Amphiphilic TEA-PAMAM dendron G3 bearing alkyl chain C18 in focal point, decorated with arginine | Decrease of CD-4 mRNA by 60% | [39] |
| Acute lymphoblastic leukemia T-cells (CCRF-CEM) | Amphiphilic Janus-type- TEA-PAMAM dendrons bearing two alkyl chains | Decrease of CD4-mRNA by 55%, knockdown of CD4 protein expression by 80% | [85] | |
| Cocktail of HIV-1 Tat/Rev (viral integrase)/dsiRNAs | PBMC CD4+, hematopoietic stem cells CD34+ | Decrease of Tat/Rev mRNA level by 50–55%, inhibition of HIV replication in infected cells by 30–40% | ||
| Delivery of siRNA by non-PAMAM constructions | ||||
| Cocktail Bcl-2, Bcl-xL, Mcl-1 (apoptosis inhibitors)/siRNA | Human cervical cancer cells (HeLa), human acute promyelocytic cells (HL-60) | PAMAM G3, G4; carbosilane G2; phosphorous G3, G4 (comparison study) | Apoptosis induction by cocktail of 3 siRNA: carbosilane (15–20%) < PAMAM (30–40%) < phosphorous G3 (45%) << phosphorous G4 (95%) | [48,49] |
| Apolipo-protein B (ApoB)/siRNA | Mice model C57BL/6 | Poly-l-lysine G6 (KG6) | Decrease of mRNA in hepatocytes by 22% (aiApoBI) and by 50% (aiApoBII), low and very low density lipoprotein level in blood by 20–25% | [97] |
| PEPCK (glucose production regulator)/siRNA | Rat hepatocytes H4IIEC3 | Combination of KG6 (dendritic poly(L-lysine) G6) and Endo-Porter peptide | Decrease of PEPCK-mRNA by 80%, knockdown of PEPCK protein expression by 95%, blood glucose level decrease by 70% | [98] |
| OCT1 (gluconeogenesis regulator via influence on metformin)/siRNA | Decrease of OCT1-mRNA by 80%, metformin (inhibitor of gluconeogenesis) action arrest | |||
| Nef (necessary protein for HIV reproduction)/siRNA | CD4+-lymphocytes | Carbosilane (CBS) G2, G3 dendrimers | HIV-1 reproduction inhibition in vitro by 35% (G2) and by 50% (G3) | [99] |
| PBMCs | Phosphorous G4 dendrimer | HIV-1 reproduction inhibition by 60% | [100] | |
| COX2 (cyclooxygenase-2, stimulator of HIV propagation in brain)/pool of four siRNA sequences | Astroglioma cells (U87MG) | NN-16 G2 (carbosilane dendrimer) | Decrease of COX2 expression in HIV-infected cells to the level of uninfected cells | [101] |
| P24, NEF (HIV structural proteins)/siRNA | 50% inhibition of HIV-1 propagation | [50] | ||
| P24, GAG1, NEF (HIV structural proteins)/cocktail of three siRNAs | T-cell lymphoma lymphoblasts (SupT1), primary PBMCs | 35% inhibition of HIV-1 propagation | [102] | |
| Bcl-2 (apoptosis inhibitor)/siRNA | Cell Line human ovarian carcinoma (A2780) | PPI G5-PEG-LHRH conjugate | Decrease of Bcl-2 mRNA level by 75% | [103] |
| Human lung carcinoma (A549) | Decrease of Bcl-2 mRNA level by >95% | |||
| A549-derived lung carcinoma xenografts in a nude mouse model | LHRH conjugates increase accumulation of dendriplexes in tumor xenografts | |||
| microRNA (Target) | Object | Dendrimer/Dendrimer Based Construction | Effect | Ref. |
|---|---|---|---|---|
| microRNA Mimic Delivery | ||||
| miR-7 (epidermal growth factor receptor) | Human glioblastoma cells U251 | Conjugate of PAMAM folic acid (FA/PAMAM) | Decreased expression of proteins EGFR by 90%, PI3K by 50%, AKT-2 by 30% | [38] |
| Immunodeficient mouse with induced glioma | Decreased expression of proteins EGFR by 50%, AKT-2 by 60%, reduction of tumor size | |||
| duplex miR-126 (signal protein SPRED1) | Human umbilical vein endothelial cells (HUVECs) | Amphiphilic Janus-type-PAMAM dendrimer, consisting of dendron G3, bound to the penetrating peptide CR9 or targeting peptide CGGRGDS | Decrease of the level SPRED1-mRNA by 50% | [120] |
| let-7g (target is unknown) | Mice with induced aggressive hepatocarcinoma | Hybrid carbosilane dendrimer G2 with a polyamine core and thiol-containing surface groups | Inhibition of liver tumor growth in mice in vivo, let-7g expression was increased 13-fold in liver tissues after 48 h post intravenous (i.v.) injection | [121] |
| miR-34a (cMET, angiogenesis and tumori-genesis regulator), miR-93 (angiogenesis regulator factor HIF1α), miR-200c (prevents metastatic spread, pathway unknown) | Human osteosarcoma cells (Saos-2 and MG-63), SCID mice with Saos-2 derived tumors | Aminated polyglycerol dendrimer (dPG-NH2) | 2–3 fold times increase in latent phase of osteosarcoma duration in vivo | [118] |
| miR-34a (procaspase-3 and Bcl-2) | Pancreatic cancer cells (MiaPaCa-2) | PAMAM dendrimer functionalized by chondroitin sulfate on the surface (CS-PAMAM) | Decreased viability of MiaPaCa-2 cells by 35%, 6.5-fold increase of the cell fraction in the apoptosis phase in vitro | [119] |
| microRNA/siRNA (Its Target) | Object | Dendrimer/Dendrimer Based Construction | Effect | Ref. |
|---|---|---|---|---|
| siRNA | ||||
| p42 MAPK-siRNA (a protein of MAPK/ERK signaling cascade regulating transcription) + metformin | Prostate cancer cells (PCa) | EDA-PAMAM G1 | Decrease of p42-mRNA by 85%, decrease of p42 protein expression by 70%, increased cells sensitivity to metformin | [124] |
| Akt-siRNA (ovarian cancer stimulator protein) + paclitaxel | Human ovarian carcinoma cells (SKOV-3) | TEA-PAMAM G6 | Decrease of Akt-mRNA by 60%, decrease of the Akt protein expression by 40%, in cell viability decreased by 40% (dendriplex) and by 60% (dendriplex + paclitaxel) | [123] |
| SKOV-3 xenograft nude mice model | Reduction of xenograft tumor size by 2 times (dendriplex) and by 4 times (dendriplex + paclitaxel) | |||
| MVP-siRNA (major vault protein involved in breast cancer drug resistance) + doxorubicin (DOX) | Breast cancer cells (MCF-7/ADR) | EDA-PAMAM/hyaluronic acid conjugate | Significant knockdown of MVP protein expression, increased cytotoxicity of the dendriplex + DOX (IC50 = 11.3 μM) compared to DOX alone (IC50 = 48.5 μM) | [125] |
| Xenograft of MCF-7/ADR in Nude BALB/c mice | Enhanced tumor target, higher intracellular accumulation, increased blood circulating time and reduced vitrotoxicity of DOX/denpriplex co-delivery compared to DOX alone | |||
| Cocktail Bcl-2, Bcl-xL, Mcl-1 (apoptosis inhibitors) / siRNA + 5- fluorouracil | Human cervical cancer cells (HeLa) | Aminopiperidine-terminated phosphorus dendrimers G3 and G4 | Synergistic effect of two anti-cancer agents (siRNA and chemodrug), enhancement of the apopotosis induction | [126] |
| microRNA Antagonists | ||||
| antimiR-21 + 5- fluorouracil | Glioblastoma cells (U251 and LN229) | TEA-PAMAM G5 | Addition of dendriplex increase cell chemosensitivity to 5-fluorouracil | [127] |
| antimiR-21 + taxol | Decrease of miR-21 level by 90–95%, increase in cells chemosensitivity to taxol (IC50 = 60–160 nM) | [128] | ||
| antimiR-21 + 5- fluorouracil | Breast cancer cells (MCF7) | Increased chemosensitivity of cells to 5-fluorouracil, a prolonged cytotoxic effect | [129] | |
| antimiR-21 + taxol | Decreased expression of p-AKT, Bcl-2, EGFR, STAT-3 proteins, increased sensitivity of cells to taxol | [130] | ||
| antimiR-21 + temozolomide | Glioma cells (U87) | Decrease of miR-21 level by 80–90%, an increase in the sensitivity of cells to temozolomide (IC50 = 7.5 μM) | [131] | |
| antimiR-21 + temozolomide | Glioma cells (U251, LN229, U87) | Decreased expression of STAT-3 and p-STAT proteins, increased chemosensitivity of cells to temozolomide | [132] | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzmitruk, V.; Apartsin, E.; Ihnatsyeu-Kachan, A.; Abashkin, V.; Shcharbin, D.; Bryszewska, M. Dendrimers Show Promise for siRNA and microRNA Therapeutics. Pharmaceutics 2018, 10, 126. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics10030126
Dzmitruk V, Apartsin E, Ihnatsyeu-Kachan A, Abashkin V, Shcharbin D, Bryszewska M. Dendrimers Show Promise for siRNA and microRNA Therapeutics. Pharmaceutics. 2018; 10(3):126. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics10030126
Chicago/Turabian StyleDzmitruk, Volha, Evgeny Apartsin, Aliaksei Ihnatsyeu-Kachan, Viktar Abashkin, Dzmitry Shcharbin, and Maria Bryszewska. 2018. "Dendrimers Show Promise for siRNA and microRNA Therapeutics" Pharmaceutics 10, no. 3: 126. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics10030126