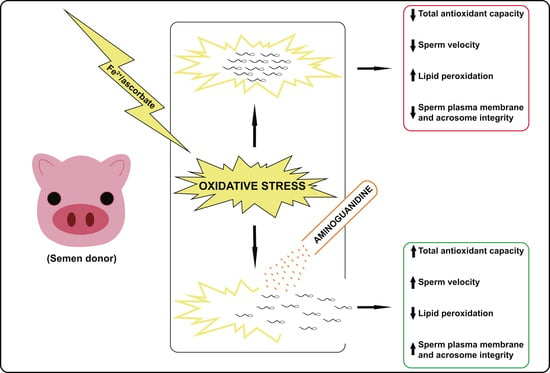
Aminoguanidine Protects Boar Spermatozoa against the Deleterious Effects of Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Processing of Sperm Samples
2.2. Assessment of Total Antioxidant Capacity
2.3. Assessment of Sperm Motility
2.4. Assessment of Lipid Peroxidation
2.5. Assessment of Sperm Plasma Membrane Integrity
2.6. Assessment of Acrosomal Status
2.7. Statistical Analysis
3. Results
3.1. Total Antioxidant Capacity
3.2. Sperm Motility and Kinetics
3.3. Lipid Peroxidation
3.4. Sperm Plasma Membrane Integrity and Acrosomal Status
4. Discussion
Supplementary Materials
Availability of Materials and Data
Author Contributions
Funding
Conflicts of Interest
References
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef]
- Giulivi, C.; Poderoso, J.J.; Boveris, A. Production of nitric oxide by mitochondria. J. Biol. Chem. 1998, 273, 11038–11043. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Saleh, R.A. Role of oxidants in male infertility: Rationale, significance, and treatment. Urol. Clin. N. Am. 2002, 29, 817–827. [Google Scholar] [CrossRef]
- Tvrdá, E.; Kňažická, Z.; Bárdos, L.; Massányi, P.; Lukáč, N. Impact of oxidative stress on male fertility—A review. Acta Vet. Hung. 2011, 59, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and consequences on oxidative stress in spermatozoa. Reprod. Fertil. Dev. 2016, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of oxidative stress on male reproduction. World J. Mens Health. 2014, 32, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guthrie, H.D.; Welch, G.R. Effects of reactive oxygen species on sperm function. Theriogenology 2012, 78, 1700–1708. [Google Scholar] [CrossRef] [PubMed]
- Knott, A.B.; Bossy-Wetzel, E. Nitric oxide in health and disease of the nervous system. Antioxid. Redox Signal. 2009, 11, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Salimian Rizi, B.; Achreja, A.; Nagrath, D. Nitric oxide: The forgotten child of tumor metabolism. Trends Cancer. 2017, 3, 659–672. [Google Scholar] [CrossRef] [PubMed]
- García-Ortiz, A.; Serrador, J.M. Nitric oxide signaling in T cell-mediated immunity. Trends Mol. Med. 2018, 24, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Helms, C.C.; Gladwin, M.T.; Kim-Shapiro, D.B. Erythrocytes and vascular function: Oxygen and nitric oxide. Front. Physiol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.L. Nitric oxide regulation of penile erection: Biology and therapeutic implications. J. Androl. 2002, 23, S20–S26. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Lamirande, E.; Gagnon, C. Nitric oxide is a signaling molecule in spermatozoa. Curr. Pharm. Des. 2003, 9, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Zweier, J.L.; Samouilov, A.; Kuppusamy, P. Non-enzymatic nitric oxide synthesis in biological systems. Biochim. Biophys. Acta. 1999, 1411, 250–262. [Google Scholar] [CrossRef] [Green Version]
- Dixit, V.D.; Parvizi, N. Nitric oxide and the control of reproduction. Anim. Reprod. Sci. 2001, 65, 1–16. [Google Scholar] [CrossRef]
- Herrero, M.B.; Pérez Martínez, S.; Viggiano, J.M.; Polak, J.M.; de Gimeno, M.F. Localization by indirect immunofluorescence of nitric oxide synthase in mouse and human spermatozoa. Reprod. Fertil. Dev. 1996, 8, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.B.; Goin, J.C.; Boquet, M.; Canteros, M.G.; Franchi, A.M.; Perez Martinez, S.; Polak, J.M.; Viggiano, J.M.; Gimeno, M.A. The nitric oxide synthase of mouse spermatozoa. FEBS Lett. 1997, 411, 39–42. [Google Scholar] [CrossRef] [Green Version]
- Aquila, S.; Giordano, F.; Guido, C.; Rago, V.; Carpino, A. Nitric oxide involvement in the acrosome reaction triggered by leptin in pig sperm. Reprod. Biol. Endocrinol. 2011, 9, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessopoulou, E.; Tomlinson, M.J.; Barratt, C.L.; Bolton, A.E.; Cooke, I.D. Origin of reactive oxygen species in human semen: Spermatozoa or leucocytes? J. Reprod. Fertil. 1992, 94, 963–970. [Google Scholar] [CrossRef]
- Gomez, E.; Buckingham, D.W.; Brindle, J.; Lanzafame, F.; Irvine, D.S.; Aitken, R.J. Development of an image analysis system to monitor the retention of residual cytoplasm by human spermatozoa: Correlation with biochemical markers of the cytoplasmic space, oxidative stress, and sperm function. J. Androl. 1996, 17, 276–287. [Google Scholar] [PubMed]
- Misko, T.P.; Moore, W.M.; Kasten, T.P.; Nickols, G.A.; Corbett, J.A.; Tilton, R.G.; McDaniel, M.L.; Williamson, J.R.; Currie, M.G. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur. J. Pharmacol. 1993, 233, 119–125. [Google Scholar] [CrossRef]
- Yildiz, G.; Demiryurek, A.T.; Sahin-Erdemli, I.; Kanzik, I. Comparison of antioxidant activities of aminoguanidine, methylguanidine and guanidine by lumino-enhanced chemiluminescence. Br. J. Pharmacol. 1998, 124, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Borg, D.J.; Forbes, J.M. Targeting advanced glycation with pharmaceutical agents: Where are we now? Glycoconj. J. 2016, 33, 653–670. [Google Scholar] [CrossRef] [PubMed]
- Gugliucci, A.; Menini, T. The polyamines spermine and spermidine protect proteins from structural and functional damage by AGE precursors: A new role for old molecules? Life Sci. 2003, 72, 2603–2616. [Google Scholar] [CrossRef]
- Jovičić, M.; Pintus, E.; Fenclová, T.; Šimonik, O.; Chmelíková, E.; Ros-Santaella, J.L.; Sedmíková, M. Effect of nitric oxide on boar sperm motility, membrane integrity, and acrosomal status during semen storage. Pol. J. Vet. Sci. 2018, 21, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Oguz, F.; Ciftci, O.; Aydın, M.; Timurkaan, N.; Beytur, A.; Altıntas, R.; Parlakpinar, H. Aminoguanidine prevents testicular damage-induced-2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in male rats. Andrologia 2013, 45, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Alizadeh, R.; Abolhassani, F.; Amidi, F.; Hassanzadeh, G.; Ejtemaei Mehr, S.; Dehpour, A.R. Aminoguanidine improves epididymal sperm parameters in varicocelized rats. Urol. Int. 2011, 86, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Alizadeh, R.; Abolhassani, F.; Amidi, F.; Ragerdi, K.I.; Fazelipour, S.; Hoshino, Y.; Sato, E.; Dehpour, A.R. Effect of aminoguanidine in sperm DNA fragmentation in varicocelized rats: Role of nitric oxide. Reprod. Sci. 2011, 18, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, N.; Abbasi, M.; Abolhassani, F.; Amidi, F.; Mahmoudi, R.; Hoshino, Y.; Sato, E.; Ragerdikashani, I. Effects of aminoguanidine on infertile varicocelized rats: A functional and morphological study. Daru J. Fac. Pharm. 2010, 18, 51–56. [Google Scholar]
- Alizadeh, R.; Navid, S.; Abbasi, N.; Yari, A.; Mazaheri, Z.; Daneshi, E.; Agarwal, A.; Abbasi, M. The effect of aminoguanidine on sperm motility and mitochondrial membrane potential in varicocelized rats. Iran. J. Basic Med. Sci. 2016, 19, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Buettner, G.R.; Jurkiewicz, B.A. Catalytic metals, ascorbate and free radicals: Combinations to avoid. Radiat. Res. 1996, 145, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Brzezińska-Ślebodzińska, E.; Ślebodziński, A.B.; Pietras, B.; Wieczorek, G. Antioxidant effect of vitamin E and glutathione on lipid peroxidation in boar semen plasma. Biol. Trace Elem. Res. 1995, 47, 69–74. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Rebolledo, Á.E.; Martínez-Pastor, F.; Fernández-Santos, M.R.; Del Olmo, E.; Bisbal, A.; Ros-Santaella, J.L.; Garde, J.J. Comparison of the TBARS assay and BODIPY C11 probes for assessing lipid peroxidation in red deer spermatozoa. Reprod. Domest. Anim. 2010, 45, e360–e368. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.P.; Vickers, S.E. Use of fluorescent probes to assess membrane integrity in mammalian spermatozoa. J. Reprod. Fertil. 1990, 88, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grieblová, A.; Pintus, E.; Ros-Santaella, J.L. Integrity of head and tail plasmalemma is associated with different kinetic variables in boar sperm. Anim. Reprod. Sci. 2017, 184, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Jeyendran, R.S.; Van der Ven, H.H.; Perez-Pelaez, M.; Crabo, B.G.; Zaneveld, L.J. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J. Reprod. Fertil. 1984, 70, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Pursel, V.G.; Johnson, L.A.; Rampacek, G.B. Acrosome morphology of boar spermatozoa incubated before cold shock. J. Anim. Sci. 1972, 34, 278–283. [Google Scholar] [CrossRef] [PubMed]
- García-Vázquez, F.A.; Hernández-Caravaca, I.; Yánez-Quintana, W.; Matás, C.; Soriano-Úbeda, C.; Izquierdo-Rico, M.J. Morphometry of boar sperm head and flagellum in semen backflow after insemination. Theriogenology 2015, 84, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Balercia, G.; Moretti, S.; Vignini, A.; Magagnini, M.; Mantero, F.; Boscaro, M.; Ricciardo-Lamonica, G.; Mazzanti, L. Role of nitric oxide concentrations on human sperm motility. J. Androl. 2004, 25, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, C.; Yildiz, O.G.; Saraymen, R.; Soyuer, S.; Kilic, E.; Ozcan, S. Aminoguanidine ameliorates radiation-induced oxidative lung damage in rats. Clin. Investig. Med. 2008, 31, 182–188. [Google Scholar] [CrossRef]
- Abraham, P.; Rabi, S.; Selvakumar, D. Protective effect of aminoguanidine against oxidative stress and bladder injury in cyclophosphamide-induced hemorrhagic cystitis in rat. Cell Biochem. Funct. 2009, 27, 56–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abo-Salem, O.M. The protective effect of aminoguanidine on doxorubicin-induced nephropathy in rats. J. Biochem. Mol. Toxicol. 2012, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, H.D.; Welch, G.R. Use of fluorescence-activated flow cytometry to determine membrane lipid peroxidation during hypothermic liquid storage and freeze-thawing of viable boar sperm loaded with 4, 4-difluoro-5-(4-phenyl-1,3-butadienyl)-4-bora-3a,4a-diaza- s-indacene-3-undecanoic acid. J. Anim. Sci. 2007, 85, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Zakošek Pipan, M.; Mrkun, J.; Kosec, M.; Nemec Svete, A.; Zrimšek, P. Superoxide dismutase: A predicting factor for boar semen characteristics for short-term preservation. Biomed Res. Int. 2014, 105280. [Google Scholar] [CrossRef] [PubMed]
- Barranco, I.; Tvarijonaviciute, A.; Perez-Patinõ, C.; Parrilla, I.; Ceron, J.J.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. High total antioxidant capacity of the porcine seminal plasma (SP-TAC) relates to sperm survival and fertility. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Khatun, A.; Rahman, M.S.; Pang, M.G. Clinical assessment of the male fertility. Obstet. Gynecol. Sci. 2018, 61, 179–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastelic, J.P.; Thundathil, J.C. Breeding soundness evaluation and semen analysis for predicting bull fertility. Reprod. Domest. Anim. 2008, 43, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Rüdiger, K.; Schulze, M. In vitro measures for assessing boar semen fertility. Reprod. Domest. Anim. 2015, 50, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Love, C.C. Modern techniques for semen evaluation. Vet. Clin. North Am. Equine Pract. 2016, 32, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Druart, X.; Gatti, J.L.; Huet, S.; Dacheux, J.L.; Humblot, P. Hypotonic resistance of boar spermatozoa: Sperm subpopulations and relationship with epididymal maturation and fertility. Reproduction 2009, 137, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M. Boar spermatozoa within the oviductal environment (II): Sperm capacitation. In Boar Reproduction: Fundamentals and New Biotechnological Trends; Bonet, S., Casas, I., Holt, W.V., Yeste, M., Eds.; Springer: Heidelberg, Germany, 2013; pp. 347–405. [Google Scholar]
- Funahashi, H. Polyspermic penetration in porcine IVM-IVF systems. Reprod. Fertil. Dev. 2003, 15, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Awda, B.J.; Mackenzie-Bell, M.; Buhr, M.M. Reactive oxygen species and boar sperm function. Biol. Reprod. 2009, 81, 553–561. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Time (h) | Total Antioxidant Capacity (mM) |
| CTR | 0 | 0.4 ± 0.1 a |
| CTR | 2 | 0.1 ± 0.0 a,A |
| CTR-ox | 2 | 0.4 ± 0.2 A |
| Ag10-ox | 2 | 2.1 ± 0.1 *** |
| Ag1-ox | 2 | 0.5 ± 0.1 |
| Ag0.1-ox | 2 | 0.4 ± 0.3 |
| CTR | 3.5 | 0.2 ± 0.0 a,A |
| CTR-ox | 3.5 | 0.2 ± 0.0 A |
| Ag10-ox | 3.5 | 2.2 ± 0.1 *** |
| Ag1-ox | 3.5 | 0.5 ± 0.1 * |
| Ag0.1-ox | 3.5 | 0.2 ± 0.1 |
| Treatment | Time (h) | TM (%) | PM (%) | VAP (µm/s) | VCL (µm/s) | VSL (µm/s) | ALH (µm) | BCF (Hz) | LIN (%) | STR (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| CTR | 0 | 77.2 ± 3.5 a | 44.9 ± 0.9 a | 40.6 ± 1.3 a | 80.8 ± 1.9 a | 30.6 ± 1.0 a | 3.0 ± 0.1 a | 13.1 ± 0.2 a | 38.0 ± 0.9 a | 75.5 ± 0.8 a |
| CTR | 2 | 63.1 ± 6.1 a,A | 62.4 ± 5.6 b,A | 39.2 ± 2.8 a,A | 67.5 ± 3.2 b,A | 36.0 ± 2.5 ab,A | 2.7 ± 0.1 b,A | 14.4 ± 0.4 b,A | 51.7 ± 1.4 b,A | 89.7 ± 0.5 b,A |
| CTR-ox | 2 | 54.2 ± 4.7 A | 61.2 ± 5.4 A | 41.4 ± 3.0 A | 68.1 ± 5.3 A | 38.9 ± 2.8 A | 2.8 ± 0.2 A | 14.7 ± 0.2 A | 55.9 ± 1.9 A | 92.3 ± 0.9 A |
| Ag10-ox | 2 | 83.0 ± 1.6 *** | 42.0 ± 2.8 *** | 42.2 ± 3.3 | 92.9 ± 8.6 *** | 30.2 ± 1.7 ** | 3.3 ± 0.3 | 12.7 ± 0.4 *** | 34.2 ± 1.8 *** | 70.8 ± 2.3 *** |
| Ag1-ox | 2 | 65.6 ± 1.4 * | 63.2 ± 2.6 | 45.0 ± 4.9 | 79.6 ± 9.4 | 40.7 ± 4.1 | 3.1 ± 0.4 | 14.0 ± 0.4 | 50.8 ± 2.3 * | 88.6 ± 1.4 * |
| Ag0.1-ox | 2 | 56.7 ± 4.7 | 58.7 ± 3.1 | 39.4 ± 4.0 | 68.4 ± 9.0 | 35.8 ± 3.1 | 2.7 ± 0.3 | 14.4 ± 0.4 | 52.5 ± 3 | 89.5 ± 2.2 |
| CTR | 3.5 | 60.0 ± 6.3 a,A | 62.8 ± 6.0 b,A | 41.5 ± 3.3 a,A | 68.9 ± 4.2 b,A | 38.6 ± 3.3 b,A | 2.7 ± 0.2 ab,A | 14.8 ± 0.4 b,A | 54.6 ± 1.9 b,A | 90.9 ± 1.0 b,A |
| CTR-ox | 3.5 | 24.9 ± 4.4 B | 43.7 ± 8.0 B | 29.4 ± 1.4 B | 45.5 ± 2.9 B | 27.9 ± 1.4 B | 1.9 ± 0.1 B | 15.1 ± 0.4 A | 62.3 ± 2.4 B | 93.6 ± 1.4 A |
| Ag10-ox | 3.5 | 69.0 ± 5.6 *** | 52.6 ± 4.0 | 36.0 ± 3.2 | 78.1 ± 6.6 *** | 29.3 ± 2.5 | 2.8 ± 0.2 *** | 12.9 ± 0.4 *** | 39.4 ± 1.6 *** | 80.5 ± 1.7 *** |
| Ag1-ox | 3.5 | 59.0 ± 3.8 *** | 63.8 ± 3.3 *** | 40.4 ± 2.5 ** | 69.2 ± 3.1 ** | 37.7 ± 2.5 ** | 2.8 ± 0.1 ** | 14.2 ± 0.4 | 54.1 ± 1.2 *** | 91.6 ± 0.6 |
| Ag0.1-ox | 3.5 | 39.6 ± 4.8 * | 53.1 ± 5.5 | 32.7 ± 1.9 | 53.0 ± 5.0 | 30.8 ± 1.5 | 2.3 ± 0.2 | 15.0 ± 0.4 | 59.1 ± 2.5 | 93.0 ± 0.8 |
| Treatment | Time (h) | Intact Head Plasma Membrane (%) | Intact Tail Plasma Membrane (%) | Normal Apical Ridge (%) | Damaged Acrosome (%) |
|---|---|---|---|---|---|
| CTR | 0 | 83.2 ± 0.6 a | 27.9 ± 2.5 a | 94.5 ± 0.3 a | 2.1 ± 0.2 a |
| CTR | 2 | 76.4 ± 0.5 b,A | 23.0 ± 4.6 ab,A | 92.1 ± 0.5 b,A | 3.2 ± 0.4 b,A |
| CTR-ox | 2 | 71.9 ± 0.7 B | 16.3 ± 4.5 A | 87.6 ± 1.3 B | 3.9 ± 0.4 A |
| Ag10-ox | 2 | 78.8 ± 0.6 *** | 24.3 ± 4.3 | 93.1 ± 0.5 *** | 2.7 ± 0.3 ** |
| Ag1-ox | 2 | 81.6 ± 1.0 *** | 20.2 ± 3.8 | 91.4 ± 1.1 *** | 2.2 ± 0.2 *** |
| Ag0.1-ox | 2 | 74.3 ± 0.3 * | 21.0 ± 4.7 | 91.1 ± 0.5 *** | 3.3 ± 0.4 |
| CTR | 3.5 | 69.3 ± 1.2 c,A | 20.4 ± 4.0 b,A | 88.7 ± 0.8 c,A | 4.7 ± 0.3 c,A |
| CTR-ox | 3.5 | 62.8 ± 0.8 B | 14.1 ± 3.7 A | 84.9 ± 1.0 B | 6.2 ± 0.3 B |
| Ag10-ox | 3.5 | 73.5 ± 0.8 *** | 22.0 ± 3.9 | 90.3 ± 1.0 *** | 4.2 ± 0.4 *** |
| Ag1-ox | 3.5 | 78.3 ± 1.1 *** | 18.1 ± 4.5 | 90.6 ± 1.1 *** | 3.1 ± 0.2 *** |
| Ag0.1-ox | 3.5 | 67.7 ± 1.2 *** | 16.0 ± 4.61 | 90.5 ± 0.6 *** | 5.3 ± 0.3 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pintus, E.; Kadlec, M.; Jovičić, M.; Sedmíková, M.; Ros-Santaella, J.L. Aminoguanidine Protects Boar Spermatozoa against the Deleterious Effects of Oxidative Stress. Pharmaceutics 2018, 10, 212. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics10040212
Pintus E, Kadlec M, Jovičić M, Sedmíková M, Ros-Santaella JL. Aminoguanidine Protects Boar Spermatozoa against the Deleterious Effects of Oxidative Stress. Pharmaceutics. 2018; 10(4):212. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics10040212
Chicago/Turabian StylePintus, Eliana, Martin Kadlec, Marija Jovičić, Markéta Sedmíková, and José Luis Ros-Santaella. 2018. "Aminoguanidine Protects Boar Spermatozoa against the Deleterious Effects of Oxidative Stress" Pharmaceutics 10, no. 4: 212. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics10040212