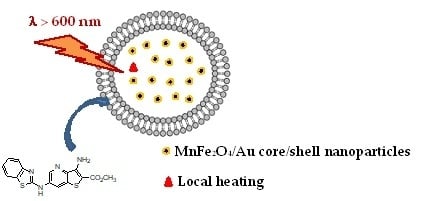
Development of Multifunctional Liposomes Containing Magnetic/Plasmonic MnFe2O4/Au Core/Shell Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Manganese Ferrite/Gold Core/Shell Nanoparticles
2.2. Preparation of Magnetoliposomes
2.3. Preparation of Giant Unilamellar Vesicles (GUVs)
2.4. Spectroscopic Measurements
2.4.1. General Methods
2.4.2. FRET Measurements
2.4.3. Fluorescence Anisotropy Measurements
2.4.4. Drug Encapsulation Efficiency
2.5. Structural Characterization
2.5.1. Transmission Electron Microscopy (TEM)
2.5.2. X-Ray Diffraction (XRD)
2.5.3. Dynamic Light Scattering (DLS)
2.6. Magnetic Measurements
2.7. Measurement of the Photothermal Effect
3. Results and Discussion
3.1. Nanoparticles Characterization
3.1.1. Absorption Spectra
3.1.2. X-Ray Diffraction (XRD) Measurements
3.1.3. Transmission Electron Microscopy (TEM)
3.2. Magnetic Properties
3.3. Magnetoliposomes as Drug Nanocarriers
3.3.1. Aqueous Magnetoliposomes
3.2.2. Solid Magnetoliposomes
3.4. Magnetoliposomes as Agents for Phototherapy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Piktel, E.; Niemirowicz, K.; Watek, M.; Wollny, T.; Deptula, P.; Bucki, R. Recent insights in nanotechnology-based drugs and formulations designed for effective anti-cancer therapy. J. Nanobiotechnol. 2016, 14, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Su, C.W.; Chiang, C.S.; Li, W.M.; Hu, S.H.; Chen, S.Y. Multifunctional nanocarriers for simultaneous encapsulation of hydrophobic and hydrophilic drugs in cancer treatment. Nanomedicine 2014, 9, 1499–1515. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Sun, S. Synthesis, Properties, and Potential Applications of Multicomponent Magnetic Nanoparticles. Adv. Funct. Mater. 2008, 18, 391–400. [Google Scholar] [CrossRef]
- Silva, S.M.; Tavallaie, R.; Sandiford, L.; Tilley, R.D.; Gooding, J.J. Gold coated magnetic nanoparticles: From preparation to surface modification for analytical and biomedical applications. Chem. Commun. 2016, 52, 7528–7540. [Google Scholar] [CrossRef] [PubMed]
- Stafford, S.; Garcia, R.S.; Gun’ku, Y.K. Multimodal Magnetic-Plasmonic Nanoparticles for Biomedical Applications. Appl. Sci. 2018, 8, 97. [Google Scholar] [CrossRef]
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ao, L.; Hu, D.; Wang, W.; Shend, Z.; Su, W. Magneto-Plasmonic Nanocapsules for Multimodal-Imaging and Magnetically Guided Combination Cancer Therapy. Chem. Mater. 2016, 28, 5896–5904. [Google Scholar] [CrossRef]
- Felton, C.; Karmakar, A.; Gartia, Y.; Ramidi, P.; Biris, A.S.; Gosh, A. Magnetic nanoparticles as contrast agents in biomedical imaging: Recent advances in iron- and manganese-based magnetic nanoparticles. Drug Metab. Rev. 2014, 46, 142–154. [Google Scholar] [CrossRef]
- Ahmas, A.; Bae, H.; Rhee, I. Highly stable silica-coated manganese ferrite nanoparticles as high-efficacy T2 contrast agents for magnetic resonance imaging. AIP Adv. 2018, 8, 55019–55028. [Google Scholar] [CrossRef]
- Kerker, M. The Scattering of Light and Other Electromagnetic Radiation, 1st ed.; Academic Press: New York, NY, USA, 1969; pp. 27–96. ISBN 978-0-12-404550-7. [Google Scholar]
- Papavassiliou, C.G. Optical properties of small inorganic and organic metal particles. Prog. Solid State Chem. 1979, 12, 185–271. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles, 1st ed.; Wiley-VCH: Weinheim, Germany, 1998; pp. 286–324. ISBN 978-0-47-129340-8. [Google Scholar]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters, 1st ed.; Springer: Heidelberg, Germany, 1995; pp. 30–68. ISBN 978-3-642-08191-0. [Google Scholar]
- Ozbay, E. Plasmonics: Merging photonics and electronics at nanoscale dimensions. Science 2006, 13, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Noguez, C. Surface Plasmons on Metal Nanoparticles: The Influence of Shape and Physical Environment. J. Phys. Chem. C 2007, 111, 3806–3819. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Govorov, A.O.; Richardson, H.H. Generating Heat with Metal Nanoparticles. Nano Today 2007, 2, 30–38. [Google Scholar] [CrossRef]
- Keblinski, P.; Cahill, D.G.; Bodapati, A.; Sullivan, C.R.; Taton, T.A. Limits of Localized Heating by Eletromagnetically Excited Nanoparticles. J. Appl. Phys. 2006, 100, 54301–54305. [Google Scholar] [CrossRef]
- Elsherbini, A.A.M.; Saber, M.; Aggag, M.; El-Shahawy, A.; Shokier, H.A.A. Laser and radiofrequency-induced hyperthermia. Int. J. Nanomed. 2011, 6, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Favazza, C.; Wang, L.V. In Vivo Photoacoustic Tomography of Chemicals: High-Resolution Functional and Molecular Optical Imaging at New Depths. Chem. Rev. 2010, 110, 2756–2782. [Google Scholar] [CrossRef] [Green Version]
- Perez-Lorenzo, M.; Vaz, B.; Salgueirin, V.; Correa-Duarte, M.A. Hollow-Shelled Nanoreactors Endowed with High Catalytic Activity. Chem. Eur. J. 2013, 19, 12196–12211. [Google Scholar] [CrossRef]
- He, Q.; Guo, S.; Qian, Z.; Chen, X. Development of Individualized Anti-Metastasis Strategies by Engineering Nanomedicines. Chem. Soc. Rev. 2015, 44, 6258–6286. [Google Scholar] [CrossRef]
- Li, Z.; Yi, S.; Cheng, L.; Yang, K.; Li, Y.; Liu, Z. Magnetic Targeting Enhanced Theranostic Strategy Based on Multimodal Imaging for Selective Ablation of Cancer. Adv. Funct. Mater. 2014, 24, 2312–2321. [Google Scholar] [CrossRef]
- Larsen, G.K.; Farr, W.; Murph, S.E.H. Multifunctional Fe2O3-Au Nanoparticles with different shapes: Enhanced catalysis, photothermal effects, and magnetic recyclability. J. Phys. Chem. C 2016, 120, 15162–15172. [Google Scholar] [CrossRef]
- Sood, A.; Arora, V.; Shah, J.; Kotnala, R.K.; Jain, T.K. Multifunctional gold coated iron oxide core-shell nanoparticles stabilizedusing thiolated sodium alginate for biomedical applications. Mater. Sci. Eng. C 2017, 80, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.R.O.; Ramos, J.M.F.; Gomes, I.T.; Almeida, B.G.; Araújo, J.P.; Queiroz, M.-J.R.P.; Coutinho, P.J.G.; Castanheira, E.M.S. Magnetoliposomes based on manganese ferrite nanoparticles as nanocarriers for anti-tumor drugs. RSC Adv. 2016, 6, 17302–17313. [Google Scholar] [CrossRef]
- Pereira, C.; Pereira, A.M.; Fernandes, C.; Rocha, M.; Mendes, R.; Garcia, M.P.F.; Guedes, A.; Tavares, P.B.; Grenèche, J.-M.; Araújo, J.P.; et al. Superparamagnetic MFe2O4 (M = Fe, Co, Mn) Nanoparticles: Tuning the Particle Size and Magnetic Properties through a Novel One-Step Coprecipitation Route. Chem. Mater. 2012, 24, 1496–1504. [Google Scholar] [CrossRef]
- Queiroz, M.-J.R.P.; Calhelha, R.C.; Vale-Silva, L.; Pinto, E.; Nascimento, M.S.-J. Novel [6-(hetero)arylamino]thieno[3,2-b]pyridines: Synthesis and anti-tumoral activities. Eur. J. Med. Chem. 2010, 45, 5732–5738. [Google Scholar] [CrossRef] [PubMed]
- Kremer, J.M.H.; Esker, M.W.J.V.D.; Pathmamanoharan, C.; Wiersema, P.H. Vesicles of variable diameter prepared by a modified injection method. Biochemistry 1977, 16, 3932–3935. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.R.O.; Gomes, I.T.; Almeida, B.G.; Araújo, J.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Magnetoliposomes based on nickel ferrite nanoparticles for biomedical applications. Phys. Chem. Chem. Phys. 2015, 17, 18011–18021. [Google Scholar] [CrossRef]
- Tamba, Y.; Terashima, H.; Yamazaki, M. A membrane filtering method for the purification of giant unilamellar vesicles. Chem. Phys. Lipids 2011, 164, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Tamba, Y.; Masum, S.M.; Yamashita, Y.; Yamazaki, M. La3+ and Gd3+ induce shape change of giant unilamellar vesicles of phosphatidylcholine. Biochim. Biophys. Acta 2002, 1564, 173–182. [Google Scholar] [CrossRef]
- Valeur, B. Molecular Fluorescence—Principles and Applications, 1st ed.; Wiley-VCH: Weinheim, Germany, 2001; pp. 247–261. ISBN 3-527-29919-X. [Google Scholar]
- Demas, J.N.; Crosby, G.A. The measurement of photoluminescence quantum yields—Review. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar] [CrossRef]
- Fery-Forgues, S.; Lavabre, D. Are fluorescence quantum yields so tricky to measure? A demonstration using familiar stationery products. J. Chem. Educ. 1999, 76, 1260–1264. [Google Scholar] [CrossRef]
- Johnson, I.; Spence, M.T.Z. Molecular Probes Handbook: A Guide to Fluorescent Probes and Labeling Technologies, 11th ed.; Life Technologies: Carlsbad, CA, USA, 2010; pp. 545–587. ISBN 978-0982927915. [Google Scholar]
- Wagner, T.; Eglinger, J. Thorstenwagner/Ij-Particlesizer: v1.0.9 Snapshot Release (Version v1.0.9-SNAPSHOT). Zenodo: Genève, Switzerland, June 2017. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Xu, X.; Liu, X. Influence of dielectric core, embedding medium and size on the optical properties of gold nanoshells. Solid State Commun. 2008, 146, 7–11. [Google Scholar] [CrossRef]
- Qian, X.; Bai, J. Theoretical Studies of the Optical Properties of Hollow Spherical Metallic Nanoshells. J. Comput. Theor. Nanosci. 2013, 10, 2354–2360. [Google Scholar] [CrossRef]
- Xu, Z.; Hou, Y.; Sun, S. Magnetic Core/Shell Fe3O4/Au and Fe3O4/Au/Ag Nanoparticles with Tunable Plasmonic Properties. J. Am. Chem. Soc. 2007, 129, 8698–8699. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Pitschke, W.; Hermann, H.; Mattern, N. The influence of surface roughness on diffracted X-ray intensities in Bragg–Brentano geometry and its effect on the structure determination by means of Rietveld analysis. Powder Diffr. 1993, 8, 74–83. [Google Scholar] [CrossRef]
- Chen, J.P.; Sorensen, C.M.; Klabunde, K.J.; Hadjipanayis, G.C.; Devlin, E.; Kostikas, A. Size-dependent magnetic properties of MnFe2O4 fine particles synthesized by coprecipitation. Phys. Rev. B 1996, 54, 9288–9296. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chang, S.-Y.; Hong, Y.-D.; Chen, S.-K.; Lin, S.-J. Anomalous decrease in X-ray diffraction intensities of Cu-Ni-Al-Co-Cr-Fe-Si alloy systems with multi-principal elements. Mat. Chem. Phys. 2007, 103, 41–46. [Google Scholar] [CrossRef]
- Neder, R.B.; Korsunskiy, V.I.; Chory, C.; Müller, G.; Hofmann, A.; Dembski, S.; Graf, C.; Rühl, E. Structural characterization of II-VI semiconductor nanoparticles. Phys. Status Solidi C 2007, 4, 3221–3233. [Google Scholar] [CrossRef]
- Rafique, M.Y.; Li-Qing, P.; Javed, Q.; Iqbal, M.Z.; Hong-Mei, Q.; Farooq, M.H.; Zhen-Gang, G.; Tanveer, M. Growth of monodisperse nanospheres of MnFe2O4 with enhanced magnetic and optical properties. Chin. Phys. B 2013, 22, 107101–107107. [Google Scholar] [CrossRef]
- Huang, J.-R.; Cheng, C. Cation and magnetic orders in MnFe2O4 from density functional calculations. J. Appl. Phys. 2013, 113, 33912–33918. [Google Scholar] [CrossRef]
- Smit, J. Magnetic Properties of Materials; McGraw Hill: New York, NY, USA, 1971; p. 89. ISBN 978-0070584457. [Google Scholar]
- Costa, C.N.C.; Hortelão, A.C.L.; Ramos, J.M.F.; Oliveira, A.D.S.; Calhelha, R.C.; Queiroz, M.-J.R.P.; Coutinho, P.J.G.; Castanheira, E.M.S. A new anti-tumoral heteroarylaminothieno[3,2-b]pyridine derivative: Its incorporation into liposomes and interaction with proteins monitored by fluorescence. Photochem. Photobiol. Sci. 2014, 13, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Sawant, R.R.; Torchilin, V.P. Challenges in development of targeted liposomal therapeutics. AAPS J. 2012, 14, 303–315. [Google Scholar] [CrossRef]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red: A selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef]
- Coutinho, P.J.G.; Castanheira, E.M.S.; Rei, M.C.; Real Oliveira, M.E.C.D. Nile Red and DCM fluorescence anisotropy studies in C12E7/DPPC mixed systems. J. Phys. Chem. B 2002, 106, 12841–12846. [Google Scholar] [CrossRef]
- Feitosa, E.; Alves, F.R.; Niemiec, A.; Oliveira, M.E.C.D.R.; Castanheira, E.M.S.; Baptista, A.L.F. Cationic liposomes in mixed didodecyldimethylammonium bromide and dioctadecyldimethylammonium bromide aqueous dispersions studied by differential scanning calorimetry, Nile Red fluorescence, and turbidity. Langmuir 2006, 22, 3579–3585. [Google Scholar] [CrossRef]
- Rodrigues, A.R.O.; Gomes, I.T.; Almeida, B.G.; Araújo, J.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Magnetoliposomes based on nickel/silica core/shell nanoparticles: Synthesis and characterization. Mater. Chem. Phys. 2014, 148, 978–987. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.R.O.; Mendes, P.M.F.; Silva, P.M.L.; Machado, V.A.; Almeida, B.G.; Araújo, J.P.; Queiroz, M.J.R.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Solid and aqueous magnetoliposomes as nanocarriers for a new potential drug active against breast cancer. Colloids Surf. B Biointerfaces 2017, 158, 460–468. [Google Scholar] [CrossRef]
- Curtis, H.; Barnes, N.S. Biology, 5th ed.; Worth Publishers: New York, NY, USA, 1989; Part 1; ISBN 978-0879013943. [Google Scholar]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Determination of the minimum temperature required for selective photothermal destruction of cancer cells with the use of immunotargeted gold nanoparticles. Photochem. Photobiol. 2006, 82, 412–417. [Google Scholar] [CrossRef]










| Sample | Ox,y,z (*) | i (*) | Micro Absorption Correction | Overall Temperature Factor, Bover | Lattice Constant (nm) | Size (nm) | Rf | χ2 |
|---|---|---|---|---|---|---|---|---|
| MnFe2O4 | 0.251 | 0.928 | No | 0 (+) | 0.84693 | 13.8 | 9.03 | 1.33 |
| MnFe2O4 | 0.251 | 0.898 | No | −2.79 | 0.84684 | 13.2 | 4.45 | 1.18 |
| MnFe2O4 | 0.257 | 0.60 | Yes (#) | 0 (+) | 0.84685 | 13.3 | 3.18 | 1.18 |
| MnFe2O4/Au | 0.257 (+) | 0.60 (+) | Yes (##) | 0 (+) | 0.84685 (+) | 13.3 | 4.70 | 1.56 |
| --- | --- | 0 (+) | 0.406945 | 11.7 | 0.68 |
| Hc (Oe) | Ms (emu/g) | Mr (emu/g) | Mr/Ms | |
|---|---|---|---|---|
| MnFe2O4/Au NPs | 13.57 | 3.15 | 0.08 | 0.03 |
| Lipid | r | |
|---|---|---|
| Liposomes [50] | Egg-PC | 0.176 |
| DOPG | 0.181 | |
| AMLs | Egg-PC | 0.173 |
| DOPG | 0.168 | |
| SMLs | DOPG | 0.175 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, A.R.O.; Matos, J.O.G.; Nova Dias, A.M.; Almeida, B.G.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Queiroz, M.-J.R.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Development of Multifunctional Liposomes Containing Magnetic/Plasmonic MnFe2O4/Au Core/Shell Nanoparticles. Pharmaceutics 2019, 11, 10. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11010010
Rodrigues ARO, Matos JOG, Nova Dias AM, Almeida BG, Pires A, Pereira AM, Araújo JP, Queiroz M-JRP, Castanheira EMS, Coutinho PJG. Development of Multifunctional Liposomes Containing Magnetic/Plasmonic MnFe2O4/Au Core/Shell Nanoparticles. Pharmaceutics. 2019; 11(1):10. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11010010
Chicago/Turabian StyleRodrigues, Ana Rita O., Joana O. G. Matos, Armando M. Nova Dias, Bernardo G. Almeida, Ana Pires, André M. Pereira, João P. Araújo, Maria-João R. P. Queiroz, Elisabete M. S. Castanheira, and Paulo J. G. Coutinho. 2019. "Development of Multifunctional Liposomes Containing Magnetic/Plasmonic MnFe2O4/Au Core/Shell Nanoparticles" Pharmaceutics 11, no. 1: 10. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11010010