Biopharmaceutical Development of a Bifonazole Multiple Emulsion for Enhanced Epidermal Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substances and Reagents
2.2. Preparation of Multiple Emulsions
2.3. Physicochemical Characterization and Stability
2.4. Rheological Properties
2.4.1. Rotational Measurements
2.4.2. Dynamic Oscillatory Measurements
2.5. Drug Content
2.5.1. Drug Extraction
2.5.2. UV Analysis
2.5.3. HPLC Analysis
2.5.4. Statistical Analyses
2.6. In Vitro Drug Release
2.6.1. Drug Release Equations
2.6.2. Statistical Analyses
2.7. Drug Permeation—Penetration
2.7.1. Drug Permeation
2.7.2. Drug Penetration
2.7.3. Statistical Analyses
2.8. Epidermal Histology
2.9. Skin Integrity Assessment
2.9.1. Subjects
2.9.2. Test Procedure
2.9.3. Skin Parameters
- -
- Skin elasticity. The effect of the formulation on the elasticity of the upper skin layers was tested with a Cutometer® MPA 580 (Courage and Khazaka, Electronic GmbH, Köln, Germany). This measurement generates a negative pressure, drawing the skin into a probe that leads to a vertical deformation. When the negative pressure is switched off, the skin recovery is characterized [31,32] in terms of skin biomechanical properties.
- -
- Corneum stratum hydration (SCH). It was performed with a Corneometer® 825 (Courage and Khazaka, Electronic GmbH, Köln, Germany). This measures the capacitance variation of the dielectric properties of epidermic stratum corneum due to changes in skin hydration.
- -
- Transepidermal Water Loss (TEWL). The retrograde water permeation through skin was measured with a Tewameter® TM 300 (Courage and Khazaka, Electronic GmbH, Köln, Germany). This measures the vapor density gradient across the skin combining temperature and relative humidity sensors located in a hollow cylinder applied on the skin surface.
2.9.4. Statistical Analyses
3. Results
3.1. Physicochemical Properties and Stability
3.2. Rheological Properties
3.2.1. Rotational Test
3.2.2. Oscillatory Test
3.3. Drug Content
3.4. Release Test
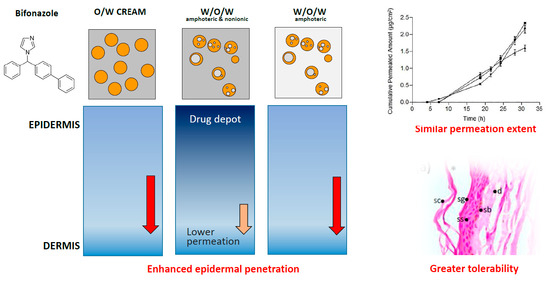
3.5. Skin Permeation
3.6. Histological Analysis
3.7. Skin Integrity Assessment
4. Discussion
4.1. Physicochemical Properties and Stability
4.2. Rheological Properties
4.3. Release and Skin Permeation
4.4. Histological Analyses
4.5. Skin Integrity
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Quatresooz, P.; Piérard-Franchimont, C.; Arrese, J.E.; Piérard, G.E. Clinicopathologic presentations of dermatomycoses in cancer patients. J. Eur. Acad. Derm. Venereol. 2008, 22, 907–917. [Google Scholar] [CrossRef] [PubMed]
- SmPC Canesmycospor®. Agencia Española de Medicamentos y Productos Sanitarios. Bayer Hispania SL. Revisited on 02/2015. Available online: https://cima.aemps.es/cima/publico/lista.html (accessed on 29 December 2018).
- Schaefer, H.; Stüttgen, G. Absolute concentrations of an antimycotic agent, Econazole, in the human skin after local application. Arzneim. Forsch. Drug Res. 1976, 26, 432–435. [Google Scholar]
- Seifriz, W. Studies in emulsion III. Double reversal of oil emulsions occasioned by the same electrolyte. J. Phys. Chem. 1925, 29, 738–749. [Google Scholar] [CrossRef]
- Grossiord, J.L.; Seiller, M. Applications. In Multiple Emulsion: Structure, Properties and Applications; Grossiord, J.L., Seiller, M., Eds.; Editions de Santé: Paris, France, 1998; p. 169. [Google Scholar]
- Nokhodchi, A.; Shokri, J.; Dashbolaghi, A.; Hassan-Zadeh, D.; Ghafourian, T.; Barzegar-Jalali, M. The enhancement effect of surfactants on the penetration of lorazepam through rat skin. Int. J. Pharm. 2003, 250, 359–369. [Google Scholar] [CrossRef]
- Firooz, A.; Nafisi, S.; Maibach, H.I. Novel drug delivery strategies for improving econazole antifungal action. Int. J. Pharm. 2015, 495, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Garti, N. Double emulsions-scope, limitations and new achievements. Colloids Surf. 1997, 123–124, 233–246. [Google Scholar] [CrossRef]
- Kaur, G.; Mehta, S.K. Developments of Polysorbate (Tween) based microemulsions: Preclinical drug delivery, toxicity and antimicrobial applications. Int. J. Pharm. 2017, 529, 134–160. [Google Scholar] [CrossRef]
- Silva, A.; Grossiord, J.L.; Puiseux, F.; Seiller, M. Insulin in W/O/W multiple emulsions: Preparation, characterization and determination of stability towards proteases in vitro. J. Microencap. 1997, 14, 311–319. [Google Scholar]
- McClements, D.J. Advances in fabrication of emulsions with enhanced functionality using structural design principles. Curr. Opin. Colloid Interface Sci. 2012, 17, 235–245. [Google Scholar] [CrossRef]
- Tang, S.Y.; Sivakumar, M.; Ng, A.M.H.; Shridharan, P. Anti-inflammatory and analgesic activity of novel oral aspirin-loaded nanoemulsion and nano multiple emulsion formulations generated using ultrasound cavitation. Int. J. Pharm. 2012, 430, 299–306. [Google Scholar] [CrossRef]
- Omotosho, J.A.; Whateley, A.T.; Florence, A.T.; Bell, G. Release of cytotoxic agents from multiple W/O/W Emulsions. J. Pharm. Pharmacol. 1987, 39, 38P. [Google Scholar]
- Nakhare, S.; Vyas, S.P. Prolonged release multiple emulsion based system bearing rifampicin: In vitro characterisation. Drug Dev. Ind. Pharm. 1995, 21, 869–878. [Google Scholar] [CrossRef]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Bachhav, Y.G.; Mondon, K.; Kalia, Y.N.; Gurny, R.; Moller, M. Novel micelle formulation to increase cutaneous bioavailability of azole antifungals. J. Control. Release 2011, 153, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Suñer, J.; Calpena, A.C.; Clares, B.; Cañadas, C.; Halbaut, L. Development of Clotrimazole Multiple W/O/W Emulsions as Vehicles for Drug Delivery: Effects of Additives on Emulsion Stability. AAPS PharmSciTech 2017, 18, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Kita, Y.; Yonezawa, D. An attempt at preparing water-in-oil-in water multiple-phase emulsions. J. Colloid Interface. Sci. 1976, 57, 353–361. [Google Scholar] [CrossRef]
- Malvern Intruments Ltd. Mastersizer 2000; User Manual. Worcestershire, UK, 2007. Available online: https://www.malvernpanalytical.com/en (accessed on 29 December 2018).
- Park, E.K.; Song, K.W. Rheological evaluation of petroleum jelly as a base material in ointment and cream formulations, steady shear flow behaviour. Arch. Pharm. Res. 2010, 33, 141–150. [Google Scholar] [CrossRef]
- Popović, G.; Čakar, M.; Agbaba, D. Determination of bifonazole in creams containing methyl- and propyl p-hydroxybenzoate by derivative spectrophotometric method. J. Pharm. Biomed. Anal. 2003, 33, 131–136. [Google Scholar] [CrossRef]
- European Medicines Agency (EMEA). Guideline on Validation of Bioanalytical Methods, Document Reference EMEA/CHMP/EWP/192217/2009; EMEA: London, UK, 2011. [Google Scholar]
- Čudina, O.A.; Čomor, M.I.; Janković, I.A. Simultaneous Determination of Bifonazole and Benzyl Alcohol in Pharmaceutical Formulations by Reverse-Phase HPLC. Chromatographia 2005, 61, 415–418. [Google Scholar] [CrossRef]
- Keshary, P.R.; Huang, Y.C.; Chien, Y.W. Mechanism of transdermal controlled nitroglycerin administration (III), Control of skin permeation rate and optimization. Drug Dev. Ind. Pharm. 1985, 11, 1213–1253. [Google Scholar] [CrossRef]
- Costa, P.; Sousa, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Yamaoka, K.; Nakagawa, T.; Uno, T. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J. Pharmacokinet. Biopharm. 1978, 6, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.A. The concept of dissolution efficiency. J. Pharm. Pharmacol. 1975, 27, 48–49. [Google Scholar] [CrossRef]
- Oestmann, E.; Lavrijsen, A.P.; Hermans, J.; Ponec, M. Skin barrier function in healthy volunteers as assessed by transepidermal water loss and vascular response to hexyl nicotinate, intra- and inter-individual variability. Br. J. Dermatol. 1993, 128, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.; Suner-Carbó, J.; Aróztegui, M.; Halbaut, L.; Barbé, C. Propuesta de protocolo de analisis sensorial en productos semisólidos: Cremas i geles. Not. Cosmet. Perfum. 2001, 260, 5–11. [Google Scholar]
- World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Bonaparte, J.P.; Ellis, D.; Chung, J. The effect of probe to skin contact force on Cutometer MPA 580 measurements. J. Med. Eng. Technol. 2013, 37, 208–212. [Google Scholar] [CrossRef]
- Neto, P.; Ferreira, M.; Bahia, F.; Costa, P. Improvement of the methods for skin mechanical properties evaluation through correlation between different techniques and factor analysis. Skin Res. Technol. 2013, 19, 405–416. [Google Scholar] [CrossRef]
- Council of Europe. The European Pharmacopoeia Commission. In European Pharmacopoeia, 8th ed.; Bifonazole monograph 04/2012:1395; Council of Europe: Strasbourg Cedex, France, 2016; p. 1670. [Google Scholar]
- Elsayed, M.A. Development of topical therapeutics for management of onychomycosis and other nail disorders, A pharmaceutical perspective. J. Control. Release 2015, 199, 132–144. [Google Scholar] [CrossRef]
- Suñer, J.; Boix, A.; Halbaut, L.; Velázquez, N.; Zamarbide, J.; Bozal-de-Febrer, N.; Calpena, A.C. Skin permeation of Econazole nitrate formulated in an enhanced hydrophilic multiple emulsion. Mycoses 2017, 60, 166–177. [Google Scholar] [CrossRef]
- Hameyer, P.; Jenni, K.R. Emulsifiers for multiple emulsions. Cosm. Toil. 1996, 111, 39–48. [Google Scholar]
- Devani, M.; Ashford, M.; Craig, D.Q.M. The emulsification and solubilisation properties of polyglycolysed oils in self-emulsifying formulations. J. Pharm. Pharmacol. 2004, 56, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Kim, Y.I.; Kim, K.M. Preparation and evaluation of aceclofenac microemulsion for transdermal delivery system. Arch. Pharm. Res. 2002, 25, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Shalaeva, M.; Kenseth, J.; Lombard, F.; Batin, A. Measurement of dissociation constants (pKa Values) of organic compounds by multiplexed capillary electrophoresis using aqueous and cosolvent buffers. J. Pharm. Sci. 2008, 97, 2581–2606. [Google Scholar] [CrossRef] [PubMed]
- El-Hadidy, G.N.; Ibrahim, H.K.; Mohamed, M.I.; El-Milligi, M.F. Microemulsions as vehicles for topical administration of voriconazole, formulation and in vitro evaluation. Drug Dev. Ind. Pharm. 2012, 38, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Muguet, V.; Seiller, M.; Barratt, G.; Ozer, O.; Marty, J.P.; Grossiord, J.L. Formulation of shear rate sensitive multiple emulsions. J. Control. Release 2001, 70, 37–49. [Google Scholar] [CrossRef]
- Tedajo, G.M.; Seiller, M.; Prognon, P.; Grossiord, J.L. pH compartmented w/o/w multiple emulsion, a diffusion study. J. Control. Release 2001, 75, 45–53. [Google Scholar] [CrossRef]
- Marku, D.; Wahlgren, M.; Rayner, M.; Sjöö, M.; Timgren, A. Characterization of starch Pickering emulsions for potential applications in topical formulations. Int. J. Pharm. 2012, 428, 1–7. [Google Scholar] [CrossRef]
- Dickinson, E.; Evison, J.; Owusu, R.K. Preparation of fine protein-stabilized water-in-oil-in water emulsions. Food Hydrocolloids 1991, 5, 481–485. [Google Scholar] [CrossRef]
- Robbe-Tomine, L.; Le Hen-Ferrenbach, C.; Pouget, T.; Tranchant, J.F. Multiple emulsions visualization methods and particle size analysis. In Multiple Emulsion: Structure, Properties and Applications; Grossiord, J.L., Seiller, M., Eds.; Editions de Sante: Paris, France, 1998. [Google Scholar]
- Celia, C.; Trapasso, E.; Cosco, D.; Paolino, D.; Fresta, M. Turbiscan® Lab Expert analysis of the stability of ethosomes and ultradeformable liposomes containing a bilayer fluidizing agent. Colloids Surf. B Biointerfaces 2009, 72, 155–160. [Google Scholar] [CrossRef]
- Vasiljevic, D.; Vuleta, G.; Primorac, M. The characterization of the semi-solid W/O/W emulsions with low concentrations of the primary polymeric emulsifier. Int. J. Cosm. Sci. 2005, 27, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Krishnaiah, Y.S.; Xu, X.; Rahman, Z.; Yang, Y.; Katragadda, U.; Lionberger, R.; Peters, J.R.; Uhl, K.; Khan, M.A. Development of performance matrix for generic product equivalence of acyclovir topical creams. Int. J. Pharm. 2014, 475, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Ruiz, J.L.; Suñer-Carbó, J.; Calpena-Campmany, A.C.; Bozal-de Febrer, N.; Halbaut-Bellowa, L.; Boix-Montañés, A.; Souto, E.B.; Clares-Naveros, B. Clotrimazole multiple W/O/W emulsion as anticandidal agent: Characterization and evaluation on skin and mucosae. Colloids Surf. B Biointerfaces 2019, 175, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Erős, I.; Csóka, I. Optimization and development of stable w/o/w cosmetic multiple emulsions by means of the Quality by Design approach. Int. J. Cosmet. Sci. 2016, 38, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, Y.; Hino, T.; Takeuchi, H.; Niwa, T.; Horibe, K. Rheological study of w/o/w emulsion by a cone-and-plate viscometer: Negative thixotropy and shear-induced phase inversion. Int. J. Pharm. 1991, 72, 65–77. [Google Scholar] [CrossRef]
- Silva, A.C.; Amaral, M.H.; Gonzalez-Mira, E.; Santos, D.; Ferreira, D. Solid lipid nanoparticles (SLN) based hydrogels as potential carriers for oral transmucosal delivery of Risperidone, Preparation and characterization studies. Colloids Surf. B Biointerfaces 2012, 93, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Valenta, C.; Schultz, K. Influence of carrageenan on the rheology and skin permeation of microemulsion formulations. J. Control. Release 2004, 95, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Rhodes, D.G.; Burgess, D.J. Multiple emulsion stability, Pressure balance and Interfacial Film strength. J. Colloid Interface Sci. 2002, 250, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, M.; Niskanen, H.; Kiesvaara, J.; Yliruusi, J. Determination of optimal combination of surfactants in creams using rheology measurements. Int. J. Pharm. 2000, 197, 143–151. [Google Scholar] [CrossRef]
- Cañadas-Enrich, C.; Abrego, G.; Alvarado, H.L.; Calpena-Campmany, A.C.; Boix-Montañes, A. Pranoprofen quantification in ex vivo corneal and scleral permeation samples: Analytical validation. J. Pharm. Biomed. Anal. 2018, 160, 109–118. [Google Scholar] [CrossRef]
- European Medicines Agency. Draft Guideline on Quality and Equivalence of Topical Products; CHMP/QWP/708282/2018; European Medicines Agency: London, UK, 2018. [Google Scholar]
- Klang, V.; Schwarz, J.C.; Haberfeld, S.; Xiao, P.; Wirth, M.; Valenta, C. Skin integrity testing and monitoring of in vitro tape stripping by capacitance-based sensor imaging. Skin Res. Technol. 2013, 19, e259–e272. [Google Scholar] [CrossRef] [PubMed]
- Fromtling, R.A. Overview of medically important antifungal azole derivatives. Clin. Microbiol. Rev. 1988, 1, 187–217. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, T.; Kodama, A.; Ryu, A.; Otagiri, M. Retention capacity of topical imidazole antifungal agents in the skin. Int. J. Pharm. 1998, 161, 195–204. [Google Scholar] [CrossRef]
- Fromtling, R.A. Bifonazole (MycosporRp) an update. Drugs Today 1985, 21, 401–404. [Google Scholar]
- Plempel, M.; Regel, E.; Büchel, K.H. Antimycotic efficacy of Bifonazole in vitro and in vivo. Arzneimittel-Forschung 1983, 33, 517–524. [Google Scholar]
- Polak, A. Antifungal activity of four antifungal drugs in the cutaneous retention time test. Sabouraudia J. Med. Vet. Mycol. 1984, 22, 501–503. [Google Scholar] [CrossRef]
- Ritter, W.; Siefert, H.M. Biological disposition and percutaneous absorption of bifonazole in animals and man. In Recent Trends in the Discovery, Development and Evaluation of Antifungal Agents; Fromtling, R.A., Ed.; JR Prous Publishers: Barcelona, Spain, 1987; pp. 383–405. [Google Scholar]
- Beggs, W.H.; Hughes, C.E. Exploitation of the direct cell damaging action of antifungal azoles. Diagn. Microb. Infect. Dis. 1987, 6, 1–3. [Google Scholar] [CrossRef]
- Patzschke, K.; Ritter, W.; Siefert, H.M.; Weber, H.; Wegner, L.A. Pharmacokietic studies following systemic and topical administration of [14C]-Bifonazole in Man. Arzneimittel-Forschung 1983, 33, 745–750. [Google Scholar]
- Sobue, S.; Sekiguchi, K. Difference in Percutaneous Absorption and Intracutaneous Distribution in Guinea Pigs among Topical Antifungal Drugs (Tioconazole Solution, Tioconazole Cream, Miconazole Nitrate Solution and Bifonazole Solution). Biol. Pharm. Bull. 2004, 27, 1428–1432. [Google Scholar] [CrossRef]
- Klang, V.; Haberfeld, S.; Hartl, A.; Valenta, C. Effect of γ-cyclodextrin on the in vitro skin permeation of a steroidal drug from nanoemulsions, Impact of experimental setup. Int. J. Pharm. 2012, 423, 535–542. [Google Scholar] [CrossRef]
- Baker, E.J.; Hadgraft, J. In vitro percutaneous absorption of arildone, a highly lipophilic drug, and the apparent no-effect of the penetration enhancer Azone in excised human skin. Pharm. Res. 1995, 12, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, D.; Matts, P.J.; Hadgraft, J.; Lane, M.E. In Vitro–In Vivo Correlation in Skin Permeation. Pharm. Res. 2014, 31, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castellano, A.; Cortell-Ivars, C.; Lopez-Carballo, G.; Herraez-Dominguez, M. The influence of Span® 20 on stratum corneum lipids in Langmuir monolayers, comparison with Azone®. Int. J. Pharm. 2000, 203, 245–253. [Google Scholar] [CrossRef]
- Salimi, A.; Hedayatipour, N.; Moghimipour, E. The Effect of Various Vehicles on the Naproxen Permeability through Rat Skin, A Mechanistic Study by DSC and FT-IR Techniques. Adv. Pharm. Bull. E 2016, 6, 9–16. [Google Scholar] [CrossRef]
- Tosti, A.; Guerra, L.; Morelli, R.; Barda, F. Prevalence and sources of sensitization to emulsifiers, a clinical study. Contact Dermat. 1990, 23, 68–72. [Google Scholar] [CrossRef]
- Coors, E.A.; Seybold, H.; Merk, H.F.; Mahler, V. Polysorbate 80 in medical products and nonimmunologic anaphylactoid reactions. Ann. Allergy Asthma Immunol. 2005, 95, 593–599. [Google Scholar] [CrossRef]
- Smits, J.; Weibel, M.; Herbst, N. Hydro-Gain®. Un Sistema humectante de origen vegetal que estimula la hidratación de la piel y fortalece la barrera lipídica. Not. Cosmet. Perfum. 2015, 342, 16–24. [Google Scholar]
- Nielsen, P.G. The Importance of the Vehicle in the Treatment of Dermatophytosis in Hereditary Palmo-Plantar Keratoderma. Mycoses 1984, 27, 227–230. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Puri, A.; Banga, A.K. Methods to simulate rubbing of topical formulation for in vitro skin permeation studies. Int. J. Pharm. 2017, 519, 22–33. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Yang, C.; Lai, C.; Zhang, C.; Liu, Y. Advantages and challenges of Tween 80 surfactant-enhanced technologies for the remediation of soils contaminated with hydrophobic organic compounds. Chem. Eng. J. 2017, 314, 98–113. [Google Scholar] [CrossRef]










| Components | Percentage Composition (w/w) | |
|---|---|---|
| JMLP01B0 | JMLP01BT | |
| Oil phase (O) | ||
| Bifonazole | 1.00 | 1.00 |
| Capric/caprylic triglyceride (Labrafac® Lipophile 1349) | 11.00 | 11.00 |
| Cetyl palmitate | 2.00 | 2.00 |
| Cetyl dimethicone copolyol (Abil® EM 90) | 1.50 | 1.50 |
| Sorbitan stearate (Span® 60) | 2.00 | 2.00 |
| Internal aqueous phase (W1) | ||
| Sodium chloride | 0.25 | 0.25 |
| Purified water at pH 6.6 | 32.25 | 32.25 |
| External aqueous phase (W2) | ||
| Carbomer (Tego® Carbomer 341) | 0.20 | 0.20 |
| Cocamidopropyl betaine (Tego® Betaine F) | 0.70 | 0.70 |
| Polysorbate 80 (Tween® 80) | - | 1.00 |
| Purified water at pH 6.6 | 49.10 | 48.10 |
| Sample | pH | Conductivity (μm/s−1) | Droplet Size Analysis (μm) | ||||
|---|---|---|---|---|---|---|---|
| D[4,3] | D[3,2] | D[v,0.1] | D[v,0.5] | D[v,0.9] | |||
| JMLP01B0 | 5.80 ± 0.04 6.01 ± 0.01 | 309.5 ± 6.5 336.3 ± 7.1 | 65.2 ± 0.8 75.2 ± 0.7 | 9.7 ± 0.2 10.5 ± 0.7 | 3.4 ± 0.3 4.1 ± 0.7 | 35.8 ± 0.3 33.8 ± 0.5 | 172.9 ± 6.3 189.2 ± 7.1 |
| JMLP01BT | 6.11 ± 0.02 6.30 ± 0.04 | 257.3 ± 3.3 289.0 ± 3.5 | 68.5 ± 1.0 60.8 ± 0.9 | 12.8 ± 0.1 12.1 ± 0.8 | 6.0 ± 0.2 6.3 ± 0.8 | 40.4 ± 5.6 49.5 ± 1.8 | 168.1 ± 3.4 165.02 ± 4.1 |
| BFZ-CF | 6.09 ± 0.04 N.A. | 199.1 ± 6.3 N.A. | 29.8 ± 9.8 N.A. | 5.8 ± 0.5 N.A. | 1.8 ± 0.2 N.A. | 18.0 ± 2.1 N.A. | 59.0 ± 13.9 N.A. |
| Formulation | Frequency (s−1) | Viscosity (mPa.s) | Oscillatory Measurements (at 24 h) | |||
|---|---|---|---|---|---|---|
| at 24 h | 180 days | G’(Pa) | G’’(Pa) | η*(Pa.s) | ||
| JMLP01B0 | 0.01 | N.A. | N.A. | 62.08 | 19.47 | 1035.00 |
| 10 | 1745.0 ± 16.7 | 1705.0 ± 15.7 | 85.09 | 27.45 | 1.42 | |
| 100 | 237.5 ± 4.7 | 205.8 ± 2.6 | N.A. | N.A. | N.A. | |
| JMLP01BT | 0.01 | N.A. | N.A. | 81.99 | 30.29 | 1391 |
| 10 | 2176.0 ± 24.6 | 2093.5 ± 19.9 | 167.60 | 60.97 | 2.84 | |
| 100 | 295.8 ± 6.8 | 261.2 ± 4.1 | N.A. | N.A. | N.A. | |
| BFZ-CF | 0.01 | N.A. | N.A. | 3736 | 1875 | 66,530 |
| 10 | 19,301.7 ± 99.7 | 19,240.5 ± 116.9 | 15,940 | 4160 | 262.20 | |
| 100 | 1901.3 ± 30.9 | 1963.0 ± 23.7 | N.A. | N.A. | N.A. | |
| Formulation | K Higuchi | AUC05/(Q5·T) | Permeation Flux | Skin Penetration | |
|---|---|---|---|---|---|
| (µg·h−1/2) | (h) | (µg/cm2h) | (µg/g) | (µg/cm2) | |
| JMLP01B0 | 539.3 ± 71.5 | 0.644 ± 0.072 | 0.137 ± 0.008 b | 13.12 ± 1.24 b | 2.87 ± 0.36 |
| JMLP01BT | 690.4 ± 97.8 a | 0.716 ± 0.026 | 0.064 ± 0.012 | 106.20 ± 8.73 | 16.60 ± 7.09 |
| BFZ-CF | 780.0 ± 87.5 | 0.692 ± 0.098 | 0.132 ± 0.009 b | 4.91 ± 0.72 b | 0.38 ± 0.07 b |
| Formulations | Skin Elasticity Mean Differences (AU) | Skin Hydration Mean Differences (AU) | TEWL Mean Differences (g/h·m2) |
|---|---|---|---|
| JMLP01B0 | 0.0521 ± 0.003 * | 8.1 ± 7.8 | 3.6 ± 1.4 |
| JMLP01BT | 0.2232 ± 0.03 | 3.6 ± 1.4 | 3.6 ± 1.9 |
| BFZ-CF | 0.1605 ± 0.04 | 6.0 ± 6.5 | 1.5 ± 2.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suñer-Carbó, J.; Calpena-Campmany, A.; Halbaut-Bellowa, L.; Clares-Naveros, B.; Rodriguez-Lagunas, M.J.; Barbolini, E.; Zamarbide-Losada, J.; Boix-Montañés, A. Biopharmaceutical Development of a Bifonazole Multiple Emulsion for Enhanced Epidermal Delivery. Pharmaceutics 2019, 11, 66. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11020066
Suñer-Carbó J, Calpena-Campmany A, Halbaut-Bellowa L, Clares-Naveros B, Rodriguez-Lagunas MJ, Barbolini E, Zamarbide-Losada J, Boix-Montañés A. Biopharmaceutical Development of a Bifonazole Multiple Emulsion for Enhanced Epidermal Delivery. Pharmaceutics. 2019; 11(2):66. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11020066
Chicago/Turabian StyleSuñer-Carbó, Joaquim, Ana Calpena-Campmany, Lyda Halbaut-Bellowa, Beatriz Clares-Naveros, María José Rodriguez-Lagunas, Elena Barbolini, Joanna Zamarbide-Losada, and Antonio Boix-Montañés. 2019. "Biopharmaceutical Development of a Bifonazole Multiple Emulsion for Enhanced Epidermal Delivery" Pharmaceutics 11, no. 2: 66. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11020066