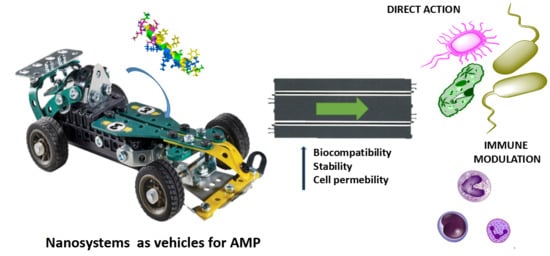
Nanosystems as Vehicles for the Delivery of Antimicrobial Peptides (AMPs)
Abstract
:1. Introduction.
2. Inorganic Nanosystems
2.1. Metal Nanoparticles
2.2. Carbon Nanotubes
3. Organic Nanosystems
3.1. Polymer Systems
3.1.1. Polymers
3.1.2. Hydrogels
3.2. Lipid-Based Systems
3.2.1. Liposomes
3.2.2. Liquid Crystalline Particles
3.3. Dendritic Systems
3.4. Cyclodextrins
3.5. Aptamer Conjugates
4. AMPs as Vehicles
5. Conclusions
Funding
Conflicts of Interest
References
- Florescu, S.A.; Calistru, P.I.; Smadu, S.; Codreanu, D.; Popescu, A.M.; Popescu, C.P.; Ceausu, E. Mortality causes in infectious diseases. Rom. J. Leg. Med. 2017, 25, 20. [Google Scholar] [CrossRef]
- Durand, G.A.; Raoult, D.; Dubourg, G. Antibiotic discovery: History, methods and perspectives. J. Antimicrob. Agents 2019, 53, 371. [Google Scholar] [CrossRef] [PubMed]
- Gajdacs, M. The concept of an ideal antibiotic: Implications for drug design. Molecules 2019, 24, 892. [Google Scholar] [CrossRef] [PubMed]
- Gandra, S.; Trett, A.; Alvarez-Uria, G.; Solomkin, J.S.; Laxminarayan, R. Is the efficacy of antibiotic prophylaxis for surgical procedures decreasing? Systematic review and meta-analysis of randomized control trials. Infect. Control. Hosp. Epidemiol. 2019, 40, 133. [Google Scholar] [CrossRef] [PubMed]
- Anesi, J.A.; Blumberg, E.A.; Abbo, L.M. Perioperative antibiotic prophylaxis to prevent surgical site infections in solid organ transplantation. Transplantation 2018, 102, 21. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, C.; Sarkar, P.; Issa, R.; Haldar, J. Alternatives to conventional antibiotics in the era of antimicrobial resistance. Trends Microbiol. 2019, 27, 323. [Google Scholar] [CrossRef] [PubMed]
- Monserrat-Martinez, A.; Gambin, Y.; Sierecki, E. Thinking outside the bug: Molecular targets and strategies to overcome antibiotic resistance. Int. J. Mol. Sci. 2019, 20, 1255. [Google Scholar] [CrossRef] [PubMed]
- Kokel, A.; Torok, M. Recent advances in the development of antimicrobial peptides (AMPs): Attempts for sustainable medicine? Curr. Med. Chem. 2018, 25, 2503. [Google Scholar] [CrossRef] [PubMed]
- Nuti, R.; Goud, N.S.; Saraswati, A.P.; Alvala, R.; Alvala, M. Antimicrobial peptides: A promising therapeutic strategy in tackling antimicrobial resistance. Curr. Med. Chem. 2017, 24, 4303. [Google Scholar] [CrossRef]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial peptides: Diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Bechinger, B.; Gorr, S.U. Antimicrobial peptides: Mechanisms of action and resistance. J. Dent. Res. 2017, 96, 254. [Google Scholar] [CrossRef] [PubMed]
- Marques-Neto, L.M.; Trentini, M.M.; das Neves, R.C.; Resende, D.P.; Procopio, V.O.; da Costa, A.C.; Kipnis, A.; Mortari, M.R.; Schwartz, E.F.; Junqueira-Kipnis, A.P. Antimicrobial and chemotactic activity of Scorpion-derived peptide, ToAP2, against Mycobacterium massiliensis. Toxins 2018, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131. [Google Scholar] [CrossRef] [PubMed]
- Greber, K.E.; Dawgul, M. Antimicrobial peptides under clinical trials. Curr. Top. Med. Chem. 2017, 17, 620. [Google Scholar] [CrossRef] [PubMed]
- Robles-Garcia, M.A.; Rodriguez-Felix, F.; Marquez-Rios, E.; Aguilar, J.A.; Barrera-Rodriguez, A.; Aguilar, J.; Ruiz-Cruz, S.; Del-Toro-Sanchez, C.L. Applications of nanotechnology in the agriculture, food and pharmaceuticals. J. Nanosci. Nanotechnol. 2016, 16, 8188. [Google Scholar] [CrossRef]
- Sanchez, F.; Sobolev, K. Nanotechnology in concrete—A review. Constr. Build. Mater. 2010, 24, 2060. [Google Scholar] [CrossRef]
- Leso, V.; Fontana, L.; Iavicoli, I. Biomedical nanotechnology: Occupational views. Nano Today 2019, 24, 10. [Google Scholar] [CrossRef]
- Fischman, M.; Murashov, V.; Borak, J.; Seward, J.; Hl, A.T.F.N. Nanotechnology and health. J. Occup. Med. 2019, 61, E95. [Google Scholar] [CrossRef]
- Knauer, N.; Pashkina, E.; Apartsin, E. Topological aspects of the design of nanocarriers for therapeutic peptides and proteins. Pharmaceutics 2019, 11, 91. [Google Scholar] [CrossRef]
- Bai, Z.M.; Wei, J.; Yu, C.M.; Han, X.S.; Qin, X.F.; Zhang, C.W.; Liao, W.Z.; Li, L.; Huang, W. Non-viral nanocarriers for intracellular delivery of microRNA therapeutics. J. Mater. Chem. 2019, 7, 1209. [Google Scholar] [CrossRef]
- Dong, P.; Rakesh, K.P.; Manukumar, H.M.; Mohammed, Y.H.E.; Karthik, C.S.; Sumathi, S.; Mallu, P.; Qin, H.L. Innovative nano-carriers in anticancer drug delivery—A comprehensive review. Bioorg. Chem. 2019, 85, 325. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Wang, P.Y.; Lin, I.C.; Huang, H.; Liu, G.S.; Tseng, C.L. Ocular drug delivery: Role of degradable polymeric nanocarriers for ophthalmic application. Int. J. Mol. Sci. 2018, 19, 2830. [Google Scholar] [CrossRef] [PubMed]
- Azharuddin, M.; Zhu, G.H.; Das, D.; Ozgur, E.; Uzun, L.; Turner, A.P.F.; Patra, H.K. A repertoire of biomedical applications of noble metal nanoparticles. Chem. Commun. 2019, 55, 6964. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Gill, G.S.; Jeet, K. Applications of Carbon Nanotubes in Drug Delivery: A Comprehensive Review. In Characterization and Biology of Nanomaterials for Drug Delivery: Nanoscience and Nanotechnology in Drug Delivery, 1st ed.; Elsevier: Amsterdam, The Netherland, 2019; 133. [Google Scholar]
- Weiner, N.; Martin, F.; Riaz, M. Liposomes as a drug delivery system. Drug Dev. Ind. Pharm. 1989, 15, 1523. [Google Scholar] [CrossRef]
- Lancelot, A.; Sierra, T.; Serrano, J.L. Nanostructured liquid-crystalline particles for drug delivery. Expert Opin. Drug Deliv. 2014, 11, 547. [Google Scholar] [CrossRef] [PubMed]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A versatile nanocarrier for drug delivery and targeting. Int. J. Pharma. 2018, 548, 707. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; George, A.; Gopi, S.; Kalarikkal, N.; Thomas, S. Polymer sutures for simultaneous wound healing and drug delivery—A review. Int. J. Pharma. 2017, 524, 454. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993. [Google Scholar] [CrossRef]
- Rajewski, R.A.; Stella, V.J. Pharmaceutical applications of cyclodextrins. 2. In vivo drug delivery. J. Pharm. Sci. 1996, 85, 1142. [Google Scholar] [CrossRef]
- Ray, P.; White, R.R. Aptamers for targeted drug delivery. Pharmaceuticals 2010, 3, 1761–1778. [Google Scholar] [CrossRef]
- Kant, T.R.; Poonam, S. Nanoparticles: Their synthesis and their applications. Res. J. Biotechnol. 2019, 14, 92. [Google Scholar]
- Strambeanu, N.; Demetrovici, L.; Dragos, D.; Lungu, M. Nanoparticles: Definition, Classification and General Physical Properties; Springer International Publishing: Basel, Switzerland, 2015. [Google Scholar]
- de Alteriis, E.; Maselli, V.; Falanga, A.; Galdiero, S.; Di Lella, F.M.; Gesuele, R.; Guida, M.; Galdiero, E. Efficiency of gold nanoparticles coated with the antimicrobial peptide Indolicidin against biofilm formation and development of Candida spp. clinical isolates. Infect. Drug Resist. 2018, 11, 915. [Google Scholar] [CrossRef] [PubMed]
- Casciaro, B.; Moros, M.; Rivera-Fernández, S.; Bellelli, A.; de la Fuente, J.M.; Mangoni, M.L. Gold-nanoparticles coated with the antimicrobial peptide Esculentin-1a(1-21)NH2 as a reliable strategy for antipseudomonal drugs. Acta Biomater. 2017, 47, 170. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.V.; Gunsolus, I.L.; Qiu, T.A.; Hurley, K.R.; Nyberg, L.H.; Frew, H.; Johnson, K.P.; Vartanian, A.M.; Jacob, L.M.; Lohse, S.E.; et al. Impacts of gold nanoparticle charge and ligand type on surface binding and toxicity to Gram-negative and Gram-positive bacteria. Chem. Sci. 2015, 6, 5186. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Robinson, S.M.; Gupta, A.; Saha, K.; Jiang, Z.; Moyano, D.F.; Sahar, A.; Riley, M.A.; Rotello, V.M. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano 2014, 8, 10682. [Google Scholar] [CrossRef] [PubMed]
- Pal, I.; Brahmkhatri, V.P.; Bera, S.; Bhattacharyya, D.; Quirishi, Y.; Bhunia, A.; Atreya, H.S. Enhanced stability and activity of an antimicrobial peptide in conjugation with silver nanoparticle. J. Colloid Interface Sci. 2016, 483, 385. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, J.; Xie, J.; Luo, Z.; Jiang, J.; Yang, Y.Y.; Liu, S. The potent antimicrobial properties of cell penetrating peptide-conjugated silver nanoparticles with excellent selectivity for Gram-positive bacteria over erythrocytes. Nanoscale 2013, 5, 3834. [Google Scholar] [CrossRef]
- Pal, I.; Bhattacharyya, D.; Kar, R.K.; Zarena, D.; Bhunia, A.; Atreya, H.S. A peptide-nanoparticle system with improved efficacy against multidrug resistant bacteria. Sci. Rep. 2019, 9, 4485. [Google Scholar] [CrossRef]
- Alberto, B.; Johan, H.; Kostas, K.; Maurizio, P.; Charalambos, D.P. Carbon nanotubes: On the road to deliver. Curr. Drug Deliv. 2005, 2, 253. [Google Scholar]
- Sur, A.; Pradhan, B.; Banerjee, A.; Aich, P. Immune activation efficacy of Indolicidin is enhanced upon conjugation with carbon nanotubes and gold nanoparticles. PLoS ONE 2015, 10, 15. [Google Scholar] [CrossRef]
- Chaudhari, A.A.; Joshi, S.; Vig, K.; Sahu, R.; Dixit, S.; Baganizi, R.; Dennis, V.A.; Singh, S.R.; Pillai, S. A three-dimensional human skin model to evaluate the inhibition of Staphylococcus aureus by antimicrobial peptide-functionalized silver carbon nanotubes. J. Biomater. Appl. 2019, 33, 924. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, A.V.; Ksenofontov, A.L.; Koksharova, O.A. Removal of antimicrobial peptides from aqueous solutions using carbon nanotubes. Nanotechnol. Russ. 2018, 13, 443. [Google Scholar] [CrossRef]
- Tkachev, A.G.; Melezhik, A.V.; Dyachkova, T.P.; Blokhin, A.N.; Burakova, E.A.; Pasko, T.V. Carbon nanomaterials of "Taunit" series: Production and application. Izv. Vyssh. Uchebn. Zaved. Khim. Khim. T. 2013, 56, 55. [Google Scholar]
- Nordström, R.; Malmsten, M. Delivery systems for antimicrobial peptides. Adv. Colloid Interface Sci. 2017, 242, 17. [Google Scholar] [CrossRef] [PubMed]
- Faya, M.; Kalhapure, R.S.; Kumalo, H.M.; Waddad, A.Y.; Omolo, C.; Govender, T. Conjugates and nano-delivery of antimicrobial peptides for enhancing therapeutic activity. J. Drug Deliv. Sci. Technol. 2018, 44, 153. [Google Scholar] [CrossRef]
- Casciaro, B.; d’Angelo, I.; Zhang, X.; Loffredo, M.R.; Conte, G.; Cappiello, F.; Quaglia, F.; Di, Y.P.P.; Ungaro, F.; Mangoni, M.L. Poly(lactide-co-glycolide) nanoparticles for prolonged therapeutic efficacy of Esculentin-1a-derived antimicrobial peptides against Pseudomonas aeruginosa lung infection: In vitro and in vivo studies. Biomacromolecules 2019, 20, 1876. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, A.; James, P.P.; Nanditha, C.K.; Kumar, G.S.V. Multiple cargo deliveries of growth factors and antimicrobial peptide using biodegradable nanopolymer as a potential wound healing system. Int. J. Nanomed. 2019, 14, 2253–2263. [Google Scholar] [CrossRef] [PubMed]
- Almaaytah, A.; Mohammed, G.K.; Abualhaijaa, A.; Al-Balas, Q. Development of novel ultrashort antimicrobial peptide nanoparticles with potent antimicrobial and antibiofilm activities against multidrug-resistant bacteria. Drug Des. Devel. Ther. 2017, 11, 3159. [Google Scholar] [CrossRef]
- Soto, K.M.; Hernández-Iturriaga, M.; Loarca-Piña, G.; Luna-Bárcenas, G.; Mendoza, S. Antimicrobial effect of Nisin electrospun amaranth: Pullulan nanofibers in apple juice and fresh cheese. Int. J. Food Microbiol. 2019, 295, 25. [Google Scholar] [CrossRef]
- Amariei, G.; Kokol, V.; Vivod, V.; Boltes, K.; Letón, P.; Rosal, R. Biocompatible antimicrobial electrospun nanofibers functionalized with ε-poly-l-lysine. Int. J. Pharm. 2018, 553, 141. [Google Scholar] [CrossRef]
- He, Y.; Jin, Y.; Wang, X.; Yao, S.; Li, Y.; Wu, Q.; Ma, G.; Cui, F.; Liu, H. An antimicrobial peptide-loaded gelatin/chitosan nanofibrous membrane fabricated by sequential layer-by-layer electrospinning and electrospraying techniques. Nanomaterials 2018, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez López, A.D.L.; Lee, M.R.; Ortiz, B.J.; Gastfriend, B.D.; Whitehead, R.; Lynn, D.M.; Palecek, S.P. Preventing S. aureus biofilm formation on titanium surfaces by the release of antimicrobial β-peptides from polyelectrolyte multilayers. Acta Biomater. 2019, 93, 50. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Jasensky, J.; Gerszberg, J.; Chen, J.; Tian, J.; Lin, T.; Lu, T.; Lahann, J.; Chen, Z. Chemically immobilized antimicrobial peptide on polymer and self-assembled monolayer substrates. Langmuir 2018, 34, 12889. [Google Scholar] [CrossRef] [PubMed]
- Muszanska, A.K.; Rochford, E.T.J.; Gruszka, A.; Bastian, A.A.; Busscher, H.J.; Norde, W.; van der Mei, H.C.; Herrmann, A. Antiadhesive polymer brush coating functionalized with antimicrobial and RGD peptides to reduce biofilm formation and enhance tissue integration. Biomacromolecules 2014, 15, 2019. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Hong, Y.; Xi, Y.; Zou, Y.; Gao, J.; Du, J. Synthesis, self-assembly, and biomedical applications of antimicrobial peptide-polymer conjugates. Biomacromolecules 2018, 19, 1701. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Andina, D.; Nahar, S.; Leroux, J.C.; Gauthier, M.A. Releasable and traceless PEGylation of arginine-rich antimicrobial peptides. Chem. Sci. 2017, 8, 4082. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.J.; Kia, A.F.A.; Hassan, F.; O’Grady, S.; Morgan, M.P.; Creaven, B.S.; McClean, S.; Harmey, J.H.; Devocelle, M. Polymeric prodrug combination to exploit the therapeutic potential of antimicrobial peptides against cancer cells. Org. Biomol. Chem. 2016, 14, 9278. [Google Scholar] [CrossRef] [PubMed]
- Golda, A.; Kosikowska-Adamus, P.; Babyak, O.; Lech, M.; Wysocka, M.; Lesner, A.; Potempa, J.; Koziel, J. Conjugate of Enkephalin and Temporin peptides as a novel therapeutic agent for sepsis. Bioconjugate Chem. 2018, 29, 4127. [Google Scholar] [CrossRef] [PubMed]
- Abbina, S.; Vappala, S.; Kumar, P.; Siren, E.M.J.; La, C.C.; Abbasi, U.; Brooks, D.E.; Kizhakkedathu, J.N. Hyperbranched polyglycerols: Recent advances in synthesis, biocompatibility and biomedical applications. J. Mater. Chem. B 2017, 5, 9249. [Google Scholar] [CrossRef]
- Kumar, P.; Shenoi, R.A.; Lai, B.F.L.; Nguyen, M.; Kizhakkedathu, J.N.; Straus, S.K. Conjugation of Aurein 2.2 to HPG yields an antimicrobial with better properties. Biomacromolecules 2015, 16, 913. [Google Scholar] [CrossRef]
- Kumar, P.; Takayesu, A.; Abbasi, U.; Kalathottukaren, M.T.; Abbina, S.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial peptide–polymer conjugates with high activity: Influence of polymer molecular weight and peptide sequence on antimicrobial activity, proteolysis, and biocompatibility. ACS Appl. Mater. Interfaces 2017, 9, 37575. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. Molecules 2019, 24, E603. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.K.; Wang, Z.J.; Xiao, Y.; Zhang, S.M.; Wang, J.L. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843. [Google Scholar] [CrossRef] [PubMed]
- Cleophas, R.T.C.; Riool, M.; van Ufford, H.C.Q.; Zaat, S.A.J.; Kruijtzer, J.A.W.; Liskamp, R.M.J. Convenient preparation of bactericidal hydrogels by covalent attachment of stabilized antimicrobial peptides using thiol-ene click chemistry. Acs Macro. Lett. 2014, 3, 477. [Google Scholar] [CrossRef]
- Cole, M.A.; Scott, T.F.; Mello, C.M. Bactericidal hydrogels via surface functionalization with Cecropin A. ACS Biomater. Sci. Eng. 2016, 2, 1894. [Google Scholar] [CrossRef]
- Nordström, R.; Nyström, L.; Andrén, O.C.J.; Malkoch, M.; Umerska, A.; Davoudi, M.; Schmidtchen, A.; Malmsten, M. Membrane interactions of microgels as carriers of antimicrobial peptides. J. Colloid Interface Sci. 2018, 513, 141. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.M.; Ju, R.J.; Liu, R.; Bu, Y.Z.; Zhang, J.Y.; Li, X.Q.; Zeng, F.; Lu, W.L. Dual-functional drug liposomes in treatment of resistant cancers. Adv. Drug Deliv. Rev. 2017, 115, 46. [Google Scholar] [CrossRef]
- Rioboo, A.; Gallego, I.; Montenegro, J. Péptidos penetrantes celulares: Descripción, mecanismo y aplicaciones. An. Quím. 2019, 115, 9. [Google Scholar]
- Raimondo, S.; Giavaresi, G.; Lorico, A.; Alessandro, R. Extracellular vesicles as biological shuttles for targeted therapies. Int. J. Mol. Sci. 2019, 20, 1848. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Kolter, M.; Wittmann, M.; Köll-Weber, M.; Süss, R. The suitability of liposomes for the delivery of hydrophobic drugs—A case study with curcumin. Eur. J. Pharm. Biopharm. 2019, 140, 20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ling, L.; Du, Y.; Yao, C.; Li, X. Reduction responsive liposomes based on paclitaxel-ss-lysophospholipid with high drug loading for intracellular delivery. Int. J. Pharm. 2019, 564, 244. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Contrast agents delivery: An up-to-date review of nanodiagnostics in neuroimaging. Nanomaterials 2019, 9, 542. [Google Scholar] [CrossRef] [PubMed]
- Narita, Y.; Shimizu, K.; Ikemoto, K.; Uchino, R.; Kosugi, M.; Maess, M.B.; Magata, Y.; Oku, N.; Ogawa, M. Macrophage-targeted, enzyme-triggered fluorescence switch-on system for detection of embolism-vulnerable atherosclerotic plaques. J. Control. Release 2019, 302, 105. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi Mohammadi, S.; Vaezi, Z.; Shojaedin-Givi, B.; Naderi-Manesh, H. Chemiluminescent liposomes as a theranostic carrier for detection of tumor cells under oxidative stress. Anal. Chim. Acta 2019, 1059, 113. [Google Scholar] [CrossRef]
- Yari, H.; Nkepang, G.; Awasthi, V. Surface modification of liposomes by a lipopolymer targeting prostate specific membrane antigen for theranostic delivery in prostate cancer. Materials 2019, 12, 756. [Google Scholar] [CrossRef]
- Chen, C.; Gao, K.; Lian, H.; Chen, C.; Yan, X. Single-particle characterization of theranostic liposomes with stimulus sensing and controlled drug release properties. Biosens. Bioelectron. 2019, 131, 185. [Google Scholar] [CrossRef]
- Grimaldi, N.; Andrade, F.; Segovia, N.; Ferrer-Tasies, L.; Sala, S.; Veciana, J.; Ventosa, N. Lipid-based nanovesicles for nanomedicine. Chem. Soc. Rev. 2016, 45, 6520. [Google Scholar] [CrossRef]
- Flühmann, B.; Ntai, I.; Borchard, G.; Simoens, S.; Mühlebach, S. Nanomedicines: The magic bullets reaching their target? Eur. J. Pharm. 2019, 128, 73. [Google Scholar] [CrossRef]
- Lamichhane, N.; Udayakumar, T.S.; D’Souza, W.D.; Simone II, C.B.; Raghavan, S.R.; Polf, J.; Mahmood, J. Liposomes: Clinical applications and potential for image-guided drug delivery. Molecules 2018, 23, 288. [Google Scholar] [CrossRef] [PubMed]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-based nanoparticles: Application and recent advances in cancer treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Ron-Doitch, S.; Sawodny, B.; Kühbacher, A.; David, M.M.N.; Samanta, A.; Phopase, J.; Burger-Kentischer, A.; Griffith, M.; Golomb, G.; Rupp, S. Reduced cytotoxicity and enhanced bioactivity of cationic antimicrobial peptides liposomes in cell cultures and 3D epidermis model against HSV. J. Control. Release 2016, 229, 163. [Google Scholar] [CrossRef] [PubMed]
- Cantor, S.; Vargas, L.; Rojas, A.; Oscar, E.; Yarce, C.J.; Salamanca, C.H.; Oñate-Garzón, J. Evaluation of the antimicrobial activity of cationic peptides loaded in surface-modified nanoliposomes against foodborne bacteria. Int. J. Mol. Sci. 2019, 20, 680. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.A.; Pinilla, C.M.B.; Brandelli, A. Pectin and polygalacturonic acid-coated liposomes as novel delivery system for Nisin: Preparation, characterization and release behavior. Food Hydrocoll. 2017, 70, 1. [Google Scholar] [CrossRef]
- Pu, C.; Tang, W. A chitosan-coated liposome encapsulating antibacterial peptide, Apep10: Characterisation, triggered-release effects and antilisterial activity in thaw water of frozen chicken. Food Funct. 2016, 7, 4310. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.M.; Boelter, J.F.; da Silveira, N.P.; Brandelli, A. Phosphatidylcholine nanovesicles coated with chitosan or chondroitin sulfate as novel devices for bacteriocin delivery. J. Nanopart. Res. 2014, 16, 2479. [Google Scholar] [CrossRef]
- Gomaa, A.I.; Martinent, C.; Hammami, R.; Fliss, I.; Subirade, M. Dual coating of liposomes as encapsulating matrix of antimicrobial peptides: Development and characterization. Front. Chem. 2017, 5, 103. [Google Scholar] [CrossRef]
- Gozdz, W.T. Cubosome topologies at various particle sizes and crystallographic symmetries. Langmuir 2015, 31, 13321. [Google Scholar] [CrossRef]
- Karami, Z.; Hamidi, M. Cubosomes: Remarkable drug delivery potential. Drug Discov. Today 2016, 21, 789. [Google Scholar] [CrossRef]
- Boge, L.; Bysell, H.; Ringstad, L.; Wennman, D.; Umerska, A.; Cassisa, V.; Eriksson, J.; Joly-Guillou, M.L.; Edwards, K.; Andersson, M. Lipid-based liquid crystals as carriers for antimicrobial peptides: Phase behavior and antimicrobial effect. Langmuir 2016, 32, 4217. [Google Scholar] [CrossRef] [PubMed]
- Boge, L.; Umerska, A.; Matougui, N.; Bysell, H.; Ringstad, L.; Davoudi, M.; Eriksson, J.; Edwards, K.; Andersson, M. Cubosomes post-loaded with antimicrobial peptides: Characterization, bactericidal effect and proteolytic stability. Int. J. Pharm. 2017, 526, 400. [Google Scholar] [CrossRef] [PubMed]
- Boge, L.; Hallstensson, K.; Ringstad, L.; Johansson, J.; Andersson, T.; Davoudi, M.; Larsson, P.T.; Mahlapuu, M.; Håkansson, J.; Andersson, M. Cubosomes for topical delivery of the antimicrobial peptide LL-37. Eur. J. Pharm. Biopharm. 2019, 134, 60. [Google Scholar] [CrossRef] [PubMed]
- Calixto, G.M.F.; Garcia, M.H.; Cilli, E.M.; Chiavacci, L.A.; Chorilli, M. Design and characterization of a novel p1025 peptide-loaded liquid crystalline system for the treatment of dental caries. Molecules 2016, 21, 158. [Google Scholar] [CrossRef] [PubMed]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Dolatabadi, J.E.N.; Hamblin, M.R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today 2018, 12, 177. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Jain, K.; Mehra, N.K.; Jain, N.K. Development and characterization of surface engineered PPI dendrimers for targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2017, 45, 414. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.J.; Kaminskas, L.M.; Karellas, P.; Krippner, G.; Lessene, R.; Porter, C.J.H. Cationic poly-L-lysine dendrimers: Pharmacokinetics, biodistribution and evidence for metabolism and bioresorption after intravenous administration to rats. Mol. Pharm. 2006, 3, 614. [Google Scholar] [CrossRef]
- Yang, S.K.; Zimmerman, S.C. Water-soluble polyglycerol dendrimers with two orthogonally reactive core functional groups for one-pot functionalization. Macromolecules 2015, 48, 2504. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, U.Y.; Han, S.C.; Kim, J.H.; Jin, S.H. Synthesis of poly(benzyl ether) dendrimers by click chemistry. Polymer-Korea 2009, 33, 67. [Google Scholar]
- Jimenez, J.L.; Gomez, R.; Briz, V.; Madrid, R.; Bryszewsk, M.; de la Mata, F.J.; Munoz-Fernandez, M.A. Carbosilane dendrimers as carriers of siRNA. J. Drug Deliv. Sci. Technol. 2012, 22, 75. [Google Scholar] [CrossRef]
- Caminade, A.M. Phosphorus dendrimers for nanomedicine. Chem. Commun. 2017, 53, 9830. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.S. Dendrimers for drug delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Acosta, G.; Pulido, D.; Maly, M.; Copa-Patino, J.L.; Soliveri, J.; Royo, M.; Gomez, R.; Albericio, F.; Ortega, P.; et al. Carbosilane dendron-peptide nanoconjugates as antimicrobial agents. Mol. Pharm. 2019, 16, 2661. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Zhang, J.X.; Wang, Z.G.; Yao, Y.J.; Han, X.; Zhao, Y.L.; Liu, J.P.; Zhang, S.Q. Identification of a cyclodextrin inclusion complex of antimicrobial peptide CM4 and its antimicrobial activity. Food Chem. 2017, 221, 296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.K.; Lyu, Y.; Zhu, X.; Wang, J.P.; Jin, Z.Y.; Narsimhan, G. Enhanced solubility and antimicrobial activity of Alamethicin in aqueous solution by complexation with γ-cyclodextrin. J. Funct. Foods 2018, 40, 700. [Google Scholar] [CrossRef]
- Teixeira, K.I.R.; Cortés, M.E.; Santos, R.A.S.; Oliveira, F.D.; Sinisterra, R.D. KR12 peptide associated with cyclodextrin: Antimicrobial and antitumor activities. Biointerphases 2016, 11, 04B307. [Google Scholar] [CrossRef]
- Ding, F.; Gao, Y.; He, X. Recent progresses in biomedical applications of aptamer-functionalized systems. Bioorg. Med. Chem. Lett. 2017, 27, 4256. [Google Scholar] [CrossRef]
- Poolsup, S.; Kim, C.Y. Therapeutic applications of synthetic nucleic acid aptamers. Curr. Opin. Biotechnol. 2017, 48, 180. [Google Scholar] [CrossRef]
- Yeom, J.H.; Lee, B.; Kim, D.; Lee, J.K.; Kim, S.; Bae, J.; Park, Y.; Lee, K. Gold nanoparticle-DNA aptamer conjugate-assisted delivery of antimicrobial peptide effectively eliminates intracellular Salmonella enterica serovar typhimurium. Biomaterials 2016, 104, 43. [Google Scholar] [CrossRef]
- Lee, B.; Park, J.; Ryu, M.; Kim, S.; Joo, M.; Yeom, J.H.; Kim, S.; Park, Y.; Lee, K.; Bae, J. Antimicrobial peptide-loaded gold nanoparticle-DNA aptamer conjugates as highly effective antibacterial therapeutics against Vibrio vulnificus. Sci. Rep. 2017, 7, 13572. [Google Scholar] [CrossRef]
- Reinhardt, A.; Neundorf, I. Design and application of antimicrobial peptide conjugates. Int. J. Mol. Sci. 2016, 17, 701. [Google Scholar] [CrossRef] [PubMed]
- Biswaro, L.S.; da Costa Sousa, M.G.; Rezende, T.M.B.; Dias, S.C.; Franco, O.L. Antimicrobial peptides and nanotechnology, recent advances and challenges. Front. Microbiol. 2018, 9, 855. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.W.; Huang, S.C.; Jin, S.L.C. A novel antimicrobial peptide-derived vehicle for oligodeoxynucleotide delivery to inhibit TNF-α expression. Int. J. Pharm. 2019, 558, 63. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, S.; Kashibe, M.; Matsumoto, K.; Hori, Y.; Kikuchi, K. Enzyme-triggered compound release using functionalized antimicrobial peptide derivatives. Chem. Sci. 2017, 8, 3047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tang, J.; Ran, R.; Liu, Y.; Zhang, Z.; Gao, H.; He, Q. Development of an anti-microbial peptide-mediated liposomal delivery system: A novel approach towards pH-responsive anti-microbial peptides. Drug Deliv. 2016, 23, 1163. [Google Scholar] [CrossRef]
- Wade, H.M.; Darling, L.E.O.; Elmore, D.E. Hybrids made from antimicrobial peptides with different mechanisms of action show enhanced membrane permeabilization. Biochim. Biophys. Acta Biomembr. 2019, 182980. [Google Scholar] [CrossRef] [PubMed]
- Manzo, G.; Serra, I.; Pira, A.; Pintus, M.; Ceccarelli, M.; Casu, M.; Rinaldi, A.C.; Scorciapino, M.A. The singular behavior of a β-type semi-synthetic two branched polypeptide: Three-dimensional structure and mode of action. Phys. Chem. Chem. Phys. 2016, 18, 30998. [Google Scholar] [CrossRef]
- Scorciapino, M.A.; Pirri, G.; Vargiu, A.V.; Ruggerone, P.; Giuliani, A.; Casu, M.; Buerck, J.; Wadhwani, P.; Ulrich, A.S.; Rinaldi, A.C. A novel dendrimeric peptide with antimicrobial properties: Structure-function analysis of SB056. Biophys. J. 2012, 102, 1039. [Google Scholar] [CrossRef]
- Bruschi, M.; Pirri, G.; Giuliani, A.; Nicoletto, S.F.; Baster, I.; Scorciapino, M.A.; Casu, M.; Rinaldi, A.C. Synthesis, characterization, antimicrobial activity and LPS-interaction properties of SB041, a novel dendrimeric peptide with antimicrobial properties. Peptides 2010, 31, 1459. [Google Scholar] [CrossRef]
- Scorciapino, M.A.; Serra, I.; Manzo, G.; Rinaldi, A.C. Antimicrobial dendrimeric peptides: Structure, activity and new therapeutic applications. Int. J. Mol. Sci. 2017, 18, 542. [Google Scholar] [CrossRef]
- Siriwardena, T.N.; Lüscher, A.; Köhler, T.; van Delden, C.; Javor, S.; Reymond, J.L. Antimicrobial peptide dendrimer chimera. Helv. Chim. Acta 2019, 102, e1900034. [Google Scholar] [CrossRef]
- Grassi, L.; Batoni, G.; Ostyn, L.; Rigole, P.; Van den Bossche, S.; Rinaldi, A.C.; Maisetta, G.; Esin, S.; Coenye, T.; Crabbé, A. The antimicrobial peptide lin-SB056-1 and its dendrimeric derivative prevent Pseudomonas aeruginosa biofilm formation in physiologically relevant models of chronic infections. Front. Microbiol. 2019, 10, 198. [Google Scholar] [CrossRef] [PubMed]


| Drug Carrier | Advantages | Disadvantages |
|---|---|---|
| Metal Nanoparticles [23] |
|
|
| Carbon nanotubes [24] |
|
|
| Liposomes [25] |
|
|
| Liquid crystalline particles [26] |
|
|
| Dendritic systems [27] |
|
|
| Polymers [28] |
|
|
| Hydrogels [29] |
|
|
| Cyclodextrins [30] |
|
|
| Aptamers [31] |
|
|
| Peptide/ Antibacterial Activity | Sequence | Delivery System | Bacterial Strain | Findings |
|---|---|---|---|---|
| Indolicidin Broad spectrum | CILPWKWPWWPWRR | AuNPs [34] | C. albicans | Biofilm formation inhibition at 24 h was 40% for indolicidin alonem and more than 50% for AuNPs–indolicidin. Eradication of 48 h biofilms with AuNPs–indolicidin was 55–65%. |
| Esculentin-1a | GIFSKLAGKKIKNLLISGLKG | AuNPs [35] | P. aeruginosa | AuNPs-Esc(1-21) preserved a concentration-dependent microbicidal effect, and killing activity was ∼12-fold increased since MBC50 was reduced from 1 for Esc(1-21) alone to 0.08 μM. Also, AuNPs-Esc(1-21) kept their antibacterial activity in the presence of trypsin. Unlike for the peptide alone, AuNPs-Esc(1-21) produced bacterial death by a membrane perturbation mechanism. |
| Odorranain-A-OA1 | VVKCSYRLGSPDSQCN | AgNPs [38] | E. coli | Bacterial death increased to 60% for the AgNP-OA1 conjugate, while it was 31% and 33% when only AgNP and peptide were added, respectively, and ∼30% when AgNP and peptide were added together but not conjugated. Conjugate cytotoxicity was evaluated in HaCaT cell line, and a higher biocompatibility (no significant IC50 values) was found compared with AgNP alone, with IC50 value of ∼96 μg/mL. |
| Andersonin-Y1 (AY1) CAY1 AY1C | FLPKLFAKITKKNMAHIR CFLPKLFAKITKKNMAHIR FLPKLFAKITKKNMAHIRC | AgNPs [40] | K. pneumoniae, P. aeruginosa, S. typhimurium | The activity of the AgNP peptide was more than the sum of the activities of the peptide and the nanoparticle taken separately. The mechanism of action was alteration of bacterial cell surface morphology followed by membrane rupture. |
| Indolicidin Broad spectrum | CILPWKWPWWPWRR | CNTs [42] | S. typhimurium | CNT conjugated indolicidin at 0.02 μg/mL protected the cell from challenge of the bacteria significantly better than free indolicidin at 20 μg/mL. |
| TP359 TP226 TP55 | MYRKKALKKD | SWCNTs-Ag [43] | S. aureus | In all cases, the conjugates presented a slight improvement of MIC where the nanotube was cargo whit 5 mg/mL of AMPs over human skin model. |
| Peptide/ Antibacterial Activity | Sequence | Delivery System | Bacterial Strain | Findings |
|---|---|---|---|---|
| Esculentin-1 Gram-negative | GIFSKLAGKKIKNLLISGLKG | PLGA NPs [48] | P. aeruginosa | Esculentin-1-loaded PLGA NPs displayed prolonged in vitro antimicrobial activity against P. aeruginosa, compared with the free peptide. Conjugated peptides led to an important reduction in the number of Pseudomona cells in the lung compared with the bacterial clearance employing the corresponding peptides in their soluble free form. |
| K4 broad-spectrum | KKKKPLFGLFFGLF | PLGA NPs [49] | S. aureus P. aeruginosa | K4 peptide and PLGA-K4 NPs killed ~75% and ~40% of S. aureus and ~50% and ~30% of P. aeruginosa, respectively. |
| Ultrashort AMP Gram-positive | RBRBR | Chitosan NPs [50] | S. aureus MRSA strains | RBRBR chitosan NPs were active against wild-type and the multidrug-resistant clinical isolated strains of Gram-positive bacteria. TRBRBR chitosan NPs presented antibiofilm activity. |
| Nisin Gram-positive | ITSISLCTPGCKTGALMGCNMKTATCHCSIHVSK | API-PUL nanofibers [51] | S. typhimurium L.monocytogenes L. mesenteroides | API-PUL nanofibers loaded 20 mg of nisin/mL. Microbial population reduction in apple juice, inactive L. mesenteroides, S. Typhimurium, and L. monocytogenes bacteria, while the nisine alone did not present antibacterial activity. |
| ε-PL broad-spectrum | 21 to 35 l-lysine residues | PAA/PVA electrospun nanofibers [52] | S. epidermidis S. aureus E. coli | The differences in antibacterial efficiency between ε-PL-functionalized and non-functionalized fibers reached one order of magnitude after 14days for liquid cultures in contact with growing cultures. |
| Pac-525 broad-spectrum | KWRRWVRWI | AMP@PLGAMS@ Gln/chitosan/nHAp [53] | S. aureus E. coli | The inhibitory ratio of the 1-week-elution solution treated with polymer-loaded system was 94.61% and 95.08% against E. coli and S. aureus, respectively. As for the 4-week-elution solution, it was 68.26% and 77.36%, respectively. |
| β-amino acid-based peptidomimetic | (ACHC-β3hVal-β3hLys)3 | Titanium surfaces with chitosan/hyaluronic acid polymer multilayers [54] | S. aureus | Improved prevention (up to 24 days) of biofilm formation on β-peptide-loaded coatings was achieved compared to uncoated substrates and films without the peptide. Release from the coatings took place over a 28-day period, and after 36 days, biofilm viability was reduced about 60% on coatings loaded with β-peptide compared to bare titanium. Minimal toxicity was observed against MC3T3-E1 cells. |
| Cecropin-melittin | KWKLFKKIGIGAVLKVLTTGLPALISC | Polymer surfaces [55] | E. coli | DBM-immobilized AMP presented similar antimicrobial activity after 5-day air exposure compared to the first day, while SAM-immobilized AMP had less antimicrobial activity the first day and no observable antimicrobial activity after 5 days. The surface-immobilized peptides killed bacteria by charge interaction and decrease in antibacterial activity is due to the loss of the peptides from the surface, maybe due to SAM decomposition, the DBM surface being more suitable. |
| - | ILPWRWPWWPWRR | Antiadhesive polymer brushes [56] | S. aureus S. epidermidis P. aeruginosa | Good antiadhesive and bactericidal properties were observed for coatings composed by PF127 polymer, PF127 modified with AMP, and PF127 modified with RGD in certain ratios, showing good tissue compatibility. |
| LL-37 derivative Broad spectrum | GFKRIVQRIKDFLRNLV | PEG [58] | E. coli | E. coli IC50 after 1 h was 40 ± 30 for the native peptide, which no longer showed antibacterial activity; 9 ± 5, 8 ± 4, and 8 ± 5 for the PEG conjugated analogue; and 20 ± 10, 20 ± 10, and 50 ± 20 for the methoxy PEG analogue, at 1, 6, and 24 h, respectively. Temporary masking of AMP arginine residues protected it against blood protease degradation and its bioactivity remained after a sustained release, which is beneficial for AMP-based therapies. |
| Aurein 2.2Δ3-cys | GLFDIVKKVVGALC | HPG [62] | S. aureus S. epidermidis | Aurein 2.2Δ3-cys antimicrobial activities, expressed as MIC, were 16 µg/mL for both S. aureus and S. epidermidis, while for or HPG-aurein 2.2Δ3-cys 2.5% conjugation ratio were 125 and 150 µg/mL, and for 5% conjugation ratio, 110 and 120 µg/mL, for S.aureus and S.epidermidis, respectively. After conjugation, antimicrobial activity decreased, so peptide density should be optimized to have activity without toxicity. |
| Cecropin A (CPA) Gram-negative | KWKLFKKIEKVGQNIRDGIIKAGPAVAVVGQATQIAK-NH2 | PEG hydrogel surfaces (PEGSH/PEGDAE formulations) [66] | S. sonnei E. coli | CPA-functionalized hydrogels antimicrobial activity against E. coli was tested. The antimicrobial behavior of immobilized CPA depended on location variation in the peptide sequence and relationship between linker type and bactericidal activity. |
| CPA-K* Gram-negative | KWKLFKKIEK VGQNIRDGII KAGPAVAVVG QATQIAKK*– | |||
| inverso-CysHHC10 Broad Spectrum | H-KRWWKWIRW-NH2 | EGDA/PTMP [67] | S. aureus S. epidermidis E. coli | 6-log reduction of bacteria of the 10 wt% AMP containing coating as compared to the blank hydrogel without AMP. |
| AP114 Gram-positive | GFGCNGPWNEDDLRCHNHCKSIKGYKGGYCAKGGFVCKCY | MAA26.5 and MAA60 microgels [68] | E. coli P. aeruginosa | Incorporated peptides can be protected from degradation by infection-related proteases at high microgel charge densities. MIC values revealed that no difference exists for E. coli when treated with AMP hydrogel or AMP alone. While, for P. aeruginosa strains, an improved MIC was observed for the DPK-060-loaded MAA26.5 microgels. For LL-37, a pronounced increase in MIC was observed as a consequence of encapsulation to the peptide into microgel. |
| LL-37 Broad spectrum | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | |||
| DPK-060 Broad spectrum | HKNKGKKNGKHNGWKWW |
| Peptide/ Antibacterial Activity | Sequence | Delivery System | Bacterial Strain | Findings |
|---|---|---|---|---|
| LL-37 | LLGDFFRSKEKIGKEFKRIVQRIKDFLRNLVPRTES | Liposomes coated with PEG [85] | Herpes simplex virus 1 (HIV-1) | Lower cytotoxicity of LL-37 liposomes was found in comparison to indolicidin liposomes. Treatment with LL-37 alone resulted in a narrow therapeutic window, with antiviral activity EC50 = 18.7 μM and cytotoxicity CC50 = 37.3 μM. However, liposomal LL-37 with EC50 = 4.2 μM and CC50 = 43.8 μM presented a wider antiviral activity at lower concentrations. |
| Indolicidin | CILPWKWPWWPWRR | |||
| Alyteserin-1c Gram-negative | GLKEIFKAGLGSLVKGIAAHVAS | Eudragit-coated liposomes [86] | E. coli | Increased antibacterial activity was observed after encapsulation and peptide chemical degradation could be prevented. MIC values for Alyteserin-1 (+2 and +5 peptide) were of 15.2 and 62.5 μM, while after coating with Eudragit liposomes, it was reduced to 1.25 and 5 μM, respectively. |
| Nisin Gram-positive | ITSISLCTPGCKTGALMGCNMKTATCHCSIHVSK | Pectin or polygalacturonic acid coated liposomes [87,89] | L. monocytogenes L. innocua Listeria sp. | The initial nisin release of coated liposomes was lower and more sustained during the first 30 h compared with that of non-coated, probably due to nisin interaction with the negatively-charged polysaccharides. Among the two coatings assayed, polygalacturonic liposomes maintained a higher antimicrobial activity after 14 days since the activities observed, first day, after 7 and 14 days, respectively were: 400, 400, and 200 AU/mL for non-coated liposomes; 800, 200, and 0 AU/mL for pectin-coated liposomes; 800, 400, and 200 AU/mL for polygalacturonic acid-coated liposomes. |
| Chitosan or chondroitin sulphate coated liposomes [89] | L. monocytogenes L. innocua Listeria sp. | The incorporation of chitosan reduced bilayer thickness giving better-organized and more stable structures, which could be related with the better maintenance of antimicrobial activity observed. Initial antibacterial activity of liposomes was the same as for nisin alone (3200 AU/mL); however, nisin lost its activity after 6 h and bacteria grew back, while at 4 and 6 h, liposomes containing nisin reduced bacteria population to almost zero. | ||
| Apep10 Gram-positive | GLARCLAGTL | Chitosan coated liposomes [88] | L. monocytogenes | Bacterial-targeted delivery was achieved, since Apep10 was only released from the chitosan-coated liposomes in presence of the LLO secreted by L. monocytogenes, a toxin that leads to pore formation in liposome formulation. Release was regulated by the extent of bacterial contamination at initial stage. |
| Microcin J25 Gram-negative | GGAGHYPEYFVGIGTPISFYG | Dual-coated pectin and whey proteins (WPI) liposomes [90] | S. enteritidis | The coating process was optimized to improve the encapsulation efficiency and the protection of microcin against gastrointestinal digestion. Double-coated (pectin/WPI) liposomes showed a significant lower degradation of microcinJ25 than that obtained with single coated or non-coated liposomes after 2 h digestion. This formulation could be suitable for colon-targeted release. |
| AP114 Gram-positive LL-37 Broad spectrum DPK-060 Broad spectrum | GFGCNGPWNEDDLRCHNHCKSIKGYKGGYCAKGGFVCKCY LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES HKNKGKKNGKHNGWKWW | Compositions: GMO or GMO/OA [93,94,95] | S. aureus P. aeruginosa E. coli A. baumannii | The antimicrobial effect of the peptide-loaded cubosomes was preserved (AP114) or sometimes even slightly enhanced (DPK-060) on S. aureus and E. coli. Cubosomes loaded with LL-37 displayed a loss in their broad-spectrum bacterial killing and were found to only have activity against Gram-negative strains. |
| p1025 Gram-positive | c-QLKTADLPAGRDETTSFVLV | Compositions: TTO, PPCA, PP and polycarbophil dispersion [96] | S. mutans | Protective effect by LLC over P1025 peptide. The conjugate preserved the anticaries and bioadhesive properties. |
| Peptide | Sequence | Delivery System | Bacterial Strain | Findings |
|---|---|---|---|---|
| AMP3 Broad spectrum | H-CRKWVWWRNR | MalG2(S(CH2)2N+Me2H·Cl−)4 [105] | S. aureus E. coli | Synergy studies showed an additive effect between carbosilane dendron and AMP3. Dendrons, AMP3, and their covalent conjugates can permeabilize bacterial membrane, causing significant morphological alterations and cellular integrity damages. |
| KR12 | KRIVQRIKDFLR | 2-hydroxypropyl-β-cyclodextrin [108] | S. mutans A. actinomycetemcomitans P. gingivalis | Antibacterial activity of the inclusion complex was enhanced, since MIC values were 7.8, 15.6, and 3.9 lg/mL for S. mutans, P. gingivalis, and A. actinomycetemcomitans, respectively. For KR12, all strains had an MIC of 7.8 lg/mL. Regarding toxicity, the complex had a lower hemolytic effect than KR12 alone, being, respectively, 48% and 73% for the maximum concentration assayed, as well as 10% and 30–40% at the lowest concentration. KR12 alone presented higher toxicity for fibroblasts (138% vs 104% LDH release), meaning that the cyclodextrin had a protective effect. |
| CM4 | GRWKIFKKIEKVGQNIRDGIVKAGPAVAVVGQAATI | β-cyclodextrin [106] | E. coli P. aeruginosa Aspergillus Niger Penicillium chrysogenum | In vitro antimicrobial activity results for the complex were similar to those for CM4. In vivo studies against P. aeruginosa performed in mice showed that the mice treated with the complex had more viability (60%) than those treated with CM4 (20%) 12 h prior to P. aeruginosa infection, and after infection, both complex and CM4 alone protected from lung injury, with complex showing higher protection efficiency by abdominal treatment. |
| Alamethicin | XPXAXAQXVXGLXPVXXEQF | γ-cyclodextrin [107] | L. monocytogenes | While alamethicin was not able to inhibit bacterial growth in aqueous medium, the complex exhibited significant antimicrobial activity, which is dependent on γ-cyclodextrin/alamethicin molar ratio. The best antimicrobial activity was found for the γ-cyclodextrin/alamethicin 5:1 mole ratio with a MIC value of 2.1875 mg/mL (4.1563 mg/mL for 10:1 complex). |
| A3-APOHis | RPDKPRPYLPRPRPPRPVRHHHHHH | AuNPs conjugated with DNA aptamer [111] | S. Typhimurium | Conjugates enhanced the bactericidal activity of A3-APOHis against intracellular bacteria by efficiently delivering it through the plasma membranes of mammalian cells and producing disruption of bacterial membrane. 100% survival of infected mice treated with the complex was observed, being the viable S. Typhimurium cells in organs ∼93–98% reduced compared with those of mice treated with buffer, A3-APOHis, AuNP-AptHis, or another peptide conjugate assayed. |
| HPA3PHis | AKKVFKRLPKLFSKIWNWKHHHHHH | AuNPs conjugated with DNA aptamer [112] | V. vulnificus | Intravenous injection of the complex led to a complete inhibition of V. vulnificus colonization in V. vulnificus-infected mice by bacterial membrane disruption, leading to 100% survival rate among the treated mice, whereas all infected mice injected with buffer, AuNP-AptHis, or HPA3PHis died before 42 h after infection. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Serrano, Á.; Gómez, R.; Ortega, P.; de la Mata, F.J. Nanosystems as Vehicles for the Delivery of Antimicrobial Peptides (AMPs). Pharmaceutics 2019, 11, 448. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11090448
Martin-Serrano Á, Gómez R, Ortega P, de la Mata FJ. Nanosystems as Vehicles for the Delivery of Antimicrobial Peptides (AMPs). Pharmaceutics. 2019; 11(9):448. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11090448
Chicago/Turabian StyleMartin-Serrano, Ángela, Rafael Gómez, Paula Ortega, and F. Javier de la Mata. 2019. "Nanosystems as Vehicles for the Delivery of Antimicrobial Peptides (AMPs)" Pharmaceutics 11, no. 9: 448. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11090448