Classification of WHO Essential Oral Medicines for Children Applying a Provisional Pediatric Biopharmaceutics Classification System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pediatric Solubility Classification
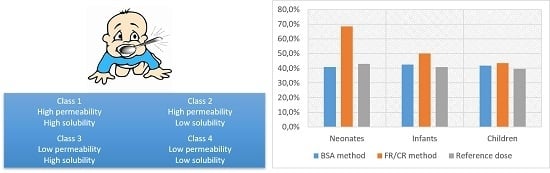
2.2. Permeability Classification
3. Results
3.1. Classification of Drug Solubility
3.2. Classification of Drug Permeability
3.3. Provisional Pediatric Biopharmaceutics Classification System (pBCS) Classification of the WHO Essential Oral Medicines for Children
3.4. Comparing Provisional Pediatric Biopharmaceutics Classification System (pBCS) with BCS in Adults
4. Discussion
4.1. Drug Solubility Classification
4.2. Drug Permeability Classification
4.3. Provisional pBCS Classification
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Drugs | Provisional pBCS | Provisional aBCS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neonate (0.5 months) | Infant (12.5 months) | Child (7 years) | ||||||||
| BSA-R | Fr-R | Ref | BSA-R | Fr-R | Ref | BSA-R | Cr-R | Ref | ||
| Drugs with unfavorable changes between aBCS and pBCS | ||||||||||
| Acetylsalicylic acid | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| Acyclovir | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| Amodiaquine | 2 | 2 | - | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| Amoxicillin | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| Benznidazole | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| Calcium gluconate | 4 | 3 | 4 | 4 | 3 | 4 | 4 | 4 | 4 | 3 |
| Cephalexin | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 1 |
| Chloramphenicol | 4 | 3 | 4 | 4 | 3 | 4 | 4 | 4 | 4 | 3 |
| Ciprofloxacin | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| Clindamycin | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 1 |
| Dexamethasone | 4 | 3 | - | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| Digoxin | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 1 |
| Enalapril | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 1 |
| Ethambutol | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Fluconazole | 4 | 3 | 4 | 4 | 3 | 4 | 4 | 4 | 4 | 3 |
| Flucytosine | 4 | 3 | 4 | 4 | 3 | 4 | 4 | 3 | 4 | 3 |
| Fludrocortisone | 3 | 3 | 4 | 3 | 3 | 4 | 3 | 3 | 4 | 3 |
| Folic acid | 3 | 3 | 4 | 3 | 3 | 4 | 3 | 3 | 3 | 3 |
| Haloperidol | 2 | 1 | - | 2 | 1 | - | 2 | 1 | 2 | 2 |
| Hydrochlorothiazide | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| Hydrocortisone | 4 | 3 | 4 | 4 | 3 | 3 | 4 | 3 | 3 | 1 |
| Mefloquine | 2 | 2 | - | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| Mercaptopurine | 4 | 4 | - | 4 | 4 | - | 4 | 4 | - | 2 |
| Methotrexate | 4 | 4 | - | 4 | 4 | - | 4 | 4 | - | 3 |
| Neostigmine | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 1 |
| Nifurtimox | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| Nystatin | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| Omeprazole | 2 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 2 | 1 |
| Phenobarbital | 4 | 3 | 4 | 3 | 3 | 4 | 3 | 3 | 4 | 3 |
| Prednisolone | 4 | 3 | - | 4 | 4 | 4 | 4 | 4 | 4 | 1 |
| Proguanil | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 1 |
| Propylthiouracil | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| Pyrazinamide | 4 | 3 | - | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| Quinine sulfate | 2 | 1 | - | 2 | 2 | - | 2 | 2 | 2 | 1 |
| Riboflavin | 4 | 3 | 4 | 4 | 3 | 4 | 3 | 3 | 4 | 3 |
| Drugs with favorable changes between aBCS and pBCS | ||||||||||
| Acetylcysteine | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Allopurinol | 4 | 3 | - | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Artesunate | 2 | 1 | - | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Azithromycin | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Cefixime | 4 | 3 | - | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Clarithromycin | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Dapsone | 4 | 3 | - | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Diazepam | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Diloxanide | 2 | 1 | - | 2 | 1 | 1 | 2 | 1 | 1 | 2 |
| Doxycycline | 4 | 3 | - | 4 | 4 | - | 4 | 4 | 4 | 4 |
| Fluoxetine | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 |
| Furosemide | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Ivermectin | 2 | 1 | - | 2 | 2 | - | 2 | 2 | 2 | 2 |
| Levofloxacin | 4 | 3 | - | 4 | 4 | - | 4 | 4 | 3 | 4 |
| Linezolid | 4 | 3 | 4 | 4 | 3 | 4 | 4 | 4 | 4 | 4 |
| Loratadine | 2 | 1 | - | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Morphine | 4 | 3 | 3 | 4 | 4 | 3 | 4 | 4 | 3 | 4 |
| Moxifloxacin | 4 | 3 | - | 4 | 4 | - | 4 | 4 | - | 4 |
| Phytomenadione | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 |
| Pyrimethamine | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 4 |
| Retinol | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Tioguanine | 4 | 3 | - | 4 | 3 | - | 4 | 3 | - | 4 |
| Trimethoprim | 4 | 3 | 4 | 4 | 3 | 4 | 4 | 4 | 4 | 4 |
| Voriconazole | 2 | 1 | - | 2 | 2 | - | 2 | 2 | 2 | 2 |
| Drugs with no changes between aBCS and pBCS | ||||||||||
| Abacavir | 3 | 3 | - | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Albendazole | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Amitriptyline | 1 | 1 | - | 1 | 1 | - | 1 | 1 | 1 | 1 |
| Ascorbic acid | 3 | 3 | - | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Atazanavir | 2 | 2 | - | 2 | 2 | - | 2 | 2 | 2 | 2 |
| Azathioprine | 4 | 4 | - | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Caffeine citrate | - | - | 3 | - | - | - | - | - | - | 3 |
| Calcium folinate | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Carbamazepine | 2 | 2 | - | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Chloroquine | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Chlorpromazine | 1 | 1 | - | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Cholecalciferol | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Cyclosporin A | 2 | 2 | - | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Clavulanic acid | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Clofazimine | 2 | 2 | - | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Cloxacillin | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Cyclizine | 4 | 4 | - | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Cyclophosphamide | 3 | 3 | - | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Cycloserine | 3 | 3 | - | 3 | 3 | - | 3 | 3 | 3 | 3 |
| Darunavir | 2 | 2 | - | 2 | 2 | - | 2 | 2 | 2 | 2 |
| Delamanid | 2 | 2 | - | 2 | 2 | - | 2 | 2 | - | 2 |
| Diethylcarbamazine | 3 | 3 | - | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Docusate sodium | 1 | 1 | - | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Efavirenz | 2 | 2 | - | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Entecavir | 3 | 3 | - | 3 | 3 | - | 3 | 3 | - | 3 |
| Ethionamide | 4 | 4 | - | 4 | 4 | - | 4 | 4 | 4 | 4 |
| Ethosuximide | 3 | 3 | - | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Etoposide | 4 | 4 | - | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Griseofulvin | 2 | 2 | - | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Hydroxycarbamide | 3 | 3 | - | 3 | 3 | - | 3 | 3 | 3 | 3 |
| Hydroxychloroquine | 1 | 1 | - | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Ibuprofen | 2 | 2 | - | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Isoniazid | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Itraconazole | 2 | 2 | - | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Lactulose | 3 | 3 | - | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Lamivudine | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Lamotrigine | 1 | 1 | - | 1 | 1 | - | 1 | 1 | 1 | 1 |
| Levamisole | 1 | 1 | - | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Levothyroxine | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Lopinavir | 2 | 2 | - | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Mebendazole | 2 | 2 | - | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Mesna | 3 | 3 | - | 3 | 3 | - | 3 | 3 | - | 3 |
| Metformin | 3 | 3 | - | 3 | 3 | - | 3 | 3 | - | 3 |
| Methadone | 1 | 1 | 1 | 1 | 1 | - | 1 | 1 | - | 1 |
| Methylprednisolone | 4 | 4 | - | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Metoclopramide | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Metronidazole | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Midazolam | 1 | 1 | - | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Miltefosine | 2 | 2 | - | 2 | 2 | - | 2 | 2 | - | 2 |
| Nevirapine | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Niclosamide | 2 | 2 | - | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Nitrofurantoin | 4 | 4 | - | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Ondansetron | 1 | 1 | - | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Oseltamivir | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Oxamniquine | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| P-amino salicylic acid | 3 | 3 | - | 3 | 3 | - | 3 | 3 | 3 | 3 |
| Paracetamol | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Phenoxymethylpenicillin potassium | 3 | 3 | - | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Phenytoin sodium | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Potassium iodide | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Praziquantel | 2 | 2 | - | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Primaquine | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Propranolol | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Pyrantel | 2 | 2 | - | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Pyridostigmine | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Pyridoxine | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Pyronaridine tetraphosphate | 3 | 3 | - | 3 | 3 | - | 3 | 3 | - | 3 |
| Raltegravir | 4 | 4 | - | 4 | 4 | - | 4 | 4 | - | 4 |
| Ranitidine | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Ribavirin | 3 | 3 | - | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Rifampicin | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Rifapentine | 2 | 2 | - | 2 | 2 | - | 2 | 2 | - | 2 |
| Ritonavir | 2 | 2 | - | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Spironolactone | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Stavudine | 3 | 3 | - | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Succimer | 3 | 3 | - | 3 | 3 | - | 3 | 3 | - | 3 |
| Sulfadiazine | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Sulfamethoxazole | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Thiamine | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Triclabendazole | 2 | 2 | - | 2 | 2 | - | 2 | 2 | 2 | 2 |
| Valganciclovir | 3 | 3 | - | 3 | 3 | - | 3 | 3 | - | 3 |
| Valproic acid | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 3 |
| Warfarin | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Zidovudine | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
References
- WHO. WHO Model Lists of Essential Medicines. Available online: http://www.who.int/medicines/publications/essentialmedicines/en/ (accessed on 19 June 2017).
- Preis, M.; Breitkreutz, J. Pediatric Drug Development and Dosage Form Design. AAPS PharmSciTech 2017, 18, 239–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kearns, G.L.; Abdel-Rahman, S.M.; Alander, S.W.; Blowey, D.L.; Leeder, J.S.; Kauffman, R.E. Developmental pharmacology—Drug disposition, action, and therapy in infants and children. N. Engl. J. Med. 2003, 349, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, H.K.; Fotaki, N.; Klein, S. Paediatric oral biopharmaceutics: Key considerations and current challenges. Adv. Drug Deliv. Rev. 2014, 73, 102–126. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, A.R.; Edginton, A.N.; Fotaki, N. Assessment of Age-Related Changes in Pediatric Gastrointestinal Solubility. Pharm. Res. 2016, 33, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.-M.; Bouzom, F.; Hugues, C.; Ungell, A.-L. Oral drug absorption in pediatrics: The intestinal wall, its developmental changes and current tools for predictions. Biopharm. Drug Dispos. 2016, 38, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Charoo, N.A.; Abdallah, D.B. Pediatric drug development: Formulation considerations. Drug Dev. Ind. Pharm. 2014, 40, 1283–1299. [Google Scholar] [CrossRef]
- Abdel-Rahman, S.M.; Amidon, G.L.; Kaul, A.; Lukacova, V.; Vinks, A.A.; Knipp, G.T.; Members of the BCS Task Force. Summary of the National Institute of Child Health and Human Development-best pharmaceuticals for Children Act Pediatric Formulation Initiatives Workshop-Pediatric Biopharmaceutics Classification System Working Group. Clin. Ther. 2012, 34, S11–S24. [Google Scholar] [CrossRef]
- Johnson, T.N.; Tanner, M.S.; Taylor, C.J.; Tucker, G.T. Enterocytic CYP3A4 in a paediatric population: Developmental changes and the effect of coeliac disease and cystic fibrosis. Br. J. Clin. Pharmacol. 2001, 51, 451–460. [Google Scholar] [CrossRef]
- WHO. Promoting Safety of Medicines for Children. Available online: http://www.who.int/medicines/publications/essentialmedicines/Promotion_safe_med_children.pdf (accessed on 22 February 2017).
- Zajicek, A.; Fossler, M.J.; Barrett, J.S.; Worthington, J.H.; Ternik, R.; Charkoftaki, G.; Lum, S.; Breitkreutz, J.; Baltezor, M.; Macheras, P.; et al. A report from the pediatric formulations task force: Perspectives on the state of child-friendly oral dosage forms. AAPS J. 2013, 15, 1072–1081. [Google Scholar] [CrossRef]
- Mistry, P.; Batchelor, H. Evidence of acceptability of oral paediatric medicines: A review. J. Pharm. Pharmacol. 2017, 69, 361–376. [Google Scholar] [CrossRef]
- Minghetti, P.; Pantano, D.; Gennari, C.G.M.; Casiraghi, A. Regulatory framework of pharmaceutical compounding and actual developments of legislation in Europe. Health Policy 2014, 117, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Committee for Medicinal Products for Human Use (CHMP). Anonymous Formulations of Choice for the Paediatric Population. Available online: https://www.ema.europa.eu/en/formulations-choice-paediatric-population (accessed on 18 October 2019).
- Batchelor, H.K.; Marriott, J.F. Formulations for children: Problems and solutions. Br. J. Clin. Pharmacol. 2015, 79, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, V.; Mameli, C.; Zuccotti, G.V. Paediatric pharmacology: Remember the excipients. Pharmacol. Res. 2011, 63, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Salunke, S.; Giacoia, G.; Tuleu, C. The STEP (safety and toxicity of excipients for paediatrics) database. Part 1-A need assessment study. Int. J. Pharm. 2012, 435, 101–111. [Google Scholar] [CrossRef]
- Salunke, S.; Brandys, B.; Giacoia, G.; Tuleu, C. The STEP (Safety and Toxicity of Excipients for Paediatrics) database: Part 2—The pilot version. Int. J. Pharm. 2013, 457, 310–322. [Google Scholar] [CrossRef]
- Chen, M.-L.; Amidon, G.L.; Benet, L.Z.; Lennernas, H.; Yu, L.X. The BCS, BDDCS, and regulatory guidances. Pharm. Res. 2011, 28, 1774–1778. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef]
- European Medicines Agency Guideline on the Investigation of Bioequivalence. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf (accessed on 20 July 2019).
- Food and Drug Administration Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System—Guidance for Industry. Available online: https://www.fda.gov/media/70963/download (accessed on 20 July 2019).
- Food and Drug Administration. Guidance for Industry Bioavailability and Bioequivalence Studies for Orally Administered Drug Products—General Considerations; U.S. Food and Drug Administration: Washington, DC, USA, 2003.
- Gandhi, S.V.; Rodriguez, W.; Khan, M.; Polli, J.E. Considerations for a Pediatric Biopharmaceutics Classification System (BCS): Application to five drugs. AAPS PharmSciTech 2014, 15, 601–611. [Google Scholar] [CrossRef]
- Oh, D.M.; Curl, R.L.; Amidon, G.L. Estimating the fraction dose absorbed from suspensions of poorly soluble compounds in humans: A mathematical model. Pharm. Res. 1993, 10, 264–270. [Google Scholar] [CrossRef]
- Montero-Padilla, S.; Velaga, S.; Morales, J.O. Buccal Dosage Forms: General Considerations for Pediatric Patients. AAPS PharmSciTech 2017, 18, 273–282. [Google Scholar] [CrossRef]
- Martir, J.; Flanagan, T.; Mann, J.; Fotaki, N. Recommended strategies for the oral administration of paediatric medicines with food and drinks in the context of their biopharmaceutical properties: A review. J. Pharm. Pharmacol. 2017, 69, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.; Lerman, J.; Christensen, S.; Farrow-Gillespie, A. Effects of duration of fasting on gastric fluid pH and volume in healthy children. Anesth. Analg. 1990, 71, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, H.; Ernest, T.; Flanagan, T.; Klein, S.; Turner, R.; Fotaki, N.; Storey, D. EuPFI Towards the development of a paediatric biopharmaceutics classification system: Results of a survey of experts. Int. J. Pharm. 2016, 511, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Shawahna, R. Pediatric Biopharmaceutical Classification System: Using Age-Appropriate Initial Gastric Volume. AAPS J. 2016, 18, 728–736. [Google Scholar] [CrossRef] [PubMed]
- The WHO Child Growth Standards. Available online: http://www.who.int/childgrowth/standards/en/ (accessed on 4 March 2016).
- Centers for Disease Control and Prevention (CDC). Stature-for-Age and Weight-for-Age Percentiles. 2 to 20 Years: Boys. Available online: http://www.cdc.gov/growthcharts (accessed on 20 July 2019).
- British Medical Association BNF for Children (BNFC). 2011–2012: Royal Pharmaceutical Society of Great Britain, British Medical Association; Pharmaceutical Press: London, UK, 2011. [Google Scholar]
- WHO Model Formulary for Children; World Health Organization: Geneva, Switzerland, 2010; ISBN 978 92 4 159932 0.
- WHO Model Prescribing Information: Drugs Used in Parasitic Diseases, 2nd ed.; World Health Organization: Geneva, Switzerland, 1995; ISBN 978-92-4-140104-3.
- Del Moral Sanchez, J.M.; Gonzalez-Alvarez, I.; Cerda-Revert, A.; Gonzalez-Alvarez, M.; Navarro-Ruiz, A.; Amidon, G.L.; Bermejo, M. Biopharmaceutical optimization in neglected diseases for paediatric patients by applying the provisional paediatric biopharmaceutical classification system. Br. J. Clin. Pharmacol. 2018, 84, 2231–2241. [Google Scholar] [CrossRef] [PubMed]
- WHO. The International Pharmacopoeia Fifth Edition. Available online: http://apps.who.int/phint/en/p/docf/ (accessed on 23 May 2016).
- Budavari, S. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 13th ed.; Merck: Whitehouse Station, NJ, USA, 2001; ISBN 978-0-911910-13-1. [Google Scholar]
- United States Pharmacopeia and National Formulary (USP 41-NF 36); United States Pharmacopeial Convention: Rockville, MD, USA, 2016.
- Kasim, N.A.; Whitehouse, M.; Ramachandran, C.; Bermejo, M.; Lennernäs, H.; Hussain, A.S.; Junginger, H.E.; Stavchansky, S.A.; Midha, K.K.; Shah, V.P.; et al. Molecular properties of WHO essential drugs and provisional biopharmaceutical classification. Mol. Pharm. 2004, 1, 85–96. [Google Scholar] [CrossRef]
- Sugano, K.; Kansy, M.; Artursson, P.; Avdeef, A.; Bendels, S.; Di, L.; Ecker, G.F.; Faller, B.; Fischer, H.; Gerebtzoff, G.; et al. Coexistence of passive and carrier-mediated processes in drug transport. Nat. Rev. Drug Discov. 2010, 9, 597–614. [Google Scholar] [CrossRef]
- Chemicalize by ChemAxon. Available online: https://chemicalize.com/#/calculation (accessed on 20 October 2016).
- Dahan, A.; Wolk, O.; Kim, Y.H.; Ramachandran, C.; Crippen, G.M.; Takagi, T.; Bermejo, M.; Amidon, G.L. Purely in silico BCS classification: Science based quality standards for the world’s drugs. Mol. Pharm. 2013, 10, 4378–4390. [Google Scholar] [CrossRef]
- Lindenberg, M.; Kopp, S.; Dressman, J.B. Classification of orally administered drugs on the World Health Organization Model list of Essential Medicines according to the biopharmaceutics classification system. Eur. J. Pharm. Biopharm. 2004, 58, 265–278. [Google Scholar] [CrossRef]
- Guimarães, M.; Statelova, M.; Holm, R.; Reppas, C.; Symilllides, M.; Vertzoni, M.; Fotaki, N. Biopharmaceutical considerations in paediatrics with a view to the evaluation of orally administered drug products—A PEARRL review. J. Pharm. Pharmacol. 2019, 71, 603–642. [Google Scholar] [CrossRef]
- Hens, B.; Van Den Abeele, J.; Rubbens, J.; Keirsebilck, M.; Roelens, J.; Schreurs, C.; Verheyen, K.; Casteels, M.; Laekeman, G.; Augustijns, P. Evaluation of real-life dosing of oral medicines with respect to fluid and food intake in a Dutch-speaking population. J. Clin. Pharm. Ther. 2017, 42, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Van Den Abeele, J.; Rayyan, M.; Hoffman, I.; Van de Vijver, E.; Zhu, W.; Augustijns, P. Gastric fluid composition in a paediatric population: Age-dependent changes relevant for gastrointestinal drug disposition. Eur J. Pharm. Sci. 2018, 123, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Miller, J.M.; Amidon, G.L. Prediction of solubility and permeability class membership: Provisional BCS classification of the world’s top oral drugs. AAPS J. 2009, 11, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, H.; European Paediatric Formulation Initiative (EUPFI). Paediatric biopharmaceutics classification system: Current status and future decisions. Int. J. Pharm. 2014, 469, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Smits, A.; De Cock, P.; Vermeulen, A.; Allegaert, K. Physiologically based pharmacokinetic (PBPK) modeling and simulation in neonatal drug development: How clinicians can contribute. Expert. Opin. Drug Metab. Toxicol. 2019, 15, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, H.K.; Kendall, R.; Desset-Brethes, S.; Alex, R.; Ernest, T.B.; European Paediatric Formulation Initiative. Application of in vitro biopharmaceutical methods in development of immediate release oral dosage forms intended for paediatric patients. Eur. J. Pharm. Biopharm. 2013, 85, 833–842. [Google Scholar] [CrossRef]
- Kohlmann, P.; Stillhart, C.; Kuentz, M.; Parrott, N. Investigating Oral Absorption of Carbamazepine in Pediatric Populations. AAPS J. 2017, 19, 1864–1877. [Google Scholar] [CrossRef]
- Johnson, T.N.; Bonner, J.J.; Tucker, G.T.; Turner, D.B.; Jamei, M. Development and applications of a physiologically-based model of paediatric oral drug absorption. Eur. J. Pharm. Sci. 2018, 115, 57–67. [Google Scholar] [CrossRef]
- Khalil, F.; Läer, S. Physiologically based pharmacokinetic models in the prediction of oral drug exposure over the entire pediatric age range-sotalol as a model drug. AAPS J. 2014, 16, 226–239. [Google Scholar] [CrossRef]




| Descriptive Term (Solubility Definition) | Parts of Solvent Required for One Part of Solute | Solubility Range (mg/mL) | Solubility Assigned (mg/mL) |
|---|---|---|---|
| Very soluble (vs) | <1 | >1000 | 1000 |
| Freely soluble (fs) | from 1 to 10 | 100–1000 | 100 |
| Soluble (s) | from 10 to 30 | 33–100 | 33 |
| Sparingly soluble (sps) | from 30 to 100 | 10–33 | 10 |
| Slightly soluble (ss) | from 100 to 1000 | 1–10 | 1 |
| Very slightly soluble (vss) | from 1000 to 10000 | 0.1–1 | 0.1 |
| Practically insoluble (pi) | >10000 | <0.1 | 0.01 |
| Parameters | Neonate (0.5 months) | Infant (12.5 months) | Child (7 years) |
|---|---|---|---|
| P50th Weight (kg) [31,32] | 3.7 | 9.8 | 23 |
| P50th Length (cm) [31,32] | 52.5 | 77 | 122 |
| BSA (m2) | 0.23 | 0.46 | 0.88 |
| Normalized V0p (mL) | 13.96 | 36.98 | 86.79 |
| Drugs | Provisional pBCS | Provisional aBCS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neonate (0.5 months) | Infant (12.5 months) | Child (7 years) | ||||||||
| BSA-R | Fr-R | Ref | BSA-R | Fr-R | Ref | BSA-R | Cr-R | Ref | ||
| Acetylsalicylic acid | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| Acyclovir | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| Amodiaquine | 2 | 2 | - | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| Amoxicillin | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| Benznidazole | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| Calcium gluconate | 4 | 3 | 4 | 4 | 3 | 4 | 4 | 4 | 4 | 3 |
| Cephalexin | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 1 |
| Chloramphenicol | 4 | 3 | 4 | 4 | 3 | 4 | 4 | 4 | 4 | 3 |
| Ciprofloxacin | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| Clindamycin | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 1 |
| Dexamethasone | 4 | 3 | - | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| Digoxin | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 1 |
| Enalapril | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 1 |
| Ethambutol | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Fluconazole | 4 | 3 | 4 | 4 | 3 | 4 | 4 | 4 | 4 | 3 |
| Flucytosine | 4 | 3 | 4 | 4 | 3 | 4 | 4 | 3 | 4 | 3 |
| Fludrocortisone | 3 | 3 | 4 | 3 | 3 | 4 | 3 | 3 | 4 | 3 |
| Folic acid | 3 | 3 | 4 | 3 | 3 | 4 | 3 | 3 | 3 | 3 |
| Haloperidol | 2 | 1 | - | 2 | 1 | - | 2 | 1 | 2 | 2 |
| Hydrochlorothiazide | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| Hydrocortisone | 4 | 3 | 4 | 4 | 3 | 3 | 4 | 3 | 3 | 1 |
| Mefloquine | 2 | 2 | - | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| Mercaptopurine | 4 | 4 | - | 4 | 4 | - | 4 | 4 | - | 2 |
| Methotrexate | 4 | 4 | - | 4 | 4 | - | 4 | 4 | - | 3 |
| Neostigmine | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 1 |
| Nifurtimox | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| Nystatin | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| Omeprazole | 2 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 2 | 1 |
| Phenobarbital | 4 | 3 | 4 | 3 | 3 | 4 | 3 | 3 | 4 | 3 |
| Prednisolone | 4 | 3 | - | 4 | 4 | 4 | 4 | 4 | 4 | 1 |
| Proguanil | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 1 |
| Propylthiouracil | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| Pyrazinamide | 4 | 3 | - | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| Quinine sulfate | 2 | 1 | - | 2 | 2 | - | 2 | 2 | 2 | 1 |
| Riboflavin | 4 | 3 | 4 | 4 | 3 | 4 | 3 | 3 | 4 | 3 |
| Drugs | Provisional pBCS | Provisional aBCS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neonate (0.5 months) | Infant (12.5 months) | Child (7 years) | ||||||||
| BSA-R | Fr-R | Ref | BSA-R | Fr-R | Ref | BSA-R | Cr-R | Ref | ||
| Acetylcysteine | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Allopurinol | 4 | 3 | - | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Artesunate | 2 | 1 | - | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Azithromycin | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Cefixime | 4 | 3 | - | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Clarithromycin | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Dapsone | 4 | 3 | - | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Diazepam | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Diloxanide | 2 | 1 | - | 2 | 1 | 1 | 2 | 1 | 1 | 2 |
| Doxycycline | 4 | 3 | - | 4 | 4 | - | 4 | 4 | 4 | 4 |
| Fluoxetine | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 |
| Furosemide | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Ivermectin | 2 | 1 | - | 2 | 2 | - | 2 | 2 | 2 | 2 |
| Levofloxacin | 4 | 3 | - | 4 | 4 | - | 4 | 4 | 3 | 4 |
| Linezolid | 4 | 3 | 4 | 4 | 3 | 4 | 4 | 4 | 4 | 4 |
| Loratadine | 2 | 1 | - | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Morphine | 4 | 3 | 3 | 4 | 4 | 3 | 4 | 4 | 3 | 4 |
| Moxifloxacin | 4 | 3 | - | 4 | 4 | - | 4 | 4 | - | 4 |
| Phytomenadione | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 |
| Pyrimethamine | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 4 |
| Retinol | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Tioguanine | 4 | 3 | - | 4 | 3 | - | 4 | 3 | - | 4 |
| Trimethoprim | 4 | 3 | 4 | 4 | 3 | 4 | 4 | 4 | 4 | 4 |
| Voriconazole | 2 | 1 | - | 2 | 2 | - | 2 | 2 | 2 | 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
delMoral-Sanchez, J.-M.; Gonzalez-Alvarez, I.; Gonzalez-Alvarez, M.; Navarro, A.; Bermejo, M. Classification of WHO Essential Oral Medicines for Children Applying a Provisional Pediatric Biopharmaceutics Classification System. Pharmaceutics 2019, 11, 567. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11110567
delMoral-Sanchez J-M, Gonzalez-Alvarez I, Gonzalez-Alvarez M, Navarro A, Bermejo M. Classification of WHO Essential Oral Medicines for Children Applying a Provisional Pediatric Biopharmaceutics Classification System. Pharmaceutics. 2019; 11(11):567. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11110567
Chicago/Turabian StyledelMoral-Sanchez, Jose-Manuel, Isabel Gonzalez-Alvarez, Marta Gonzalez-Alvarez, Andres Navarro, and Marival Bermejo. 2019. "Classification of WHO Essential Oral Medicines for Children Applying a Provisional Pediatric Biopharmaceutics Classification System" Pharmaceutics 11, no. 11: 567. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11110567