Ultrastructural Study of Acanthamoeba polyphaga Trophozoites and Cysts Treated In Vitro with Cationic Carbosilane Dendrimers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Acanthamoeba polyphaga
2.2. Cationic Carbosilane Dendrimers
2.3. Chlorhexidine Digluconate
2.4. Scanning Electron Microscopy (SEM)
2.5. Transmission Electron Microscopy (TEM)
2.6. Statistical Analysis
3. Results
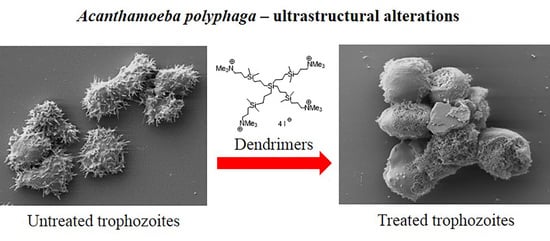
3.1. Analysis of Trophozoites Morphology
3.2. Alterations on Cysts Morphology by SEM and TEM
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hollhumer, R.; Keay, L.; Watson, S.L. Acanthamoeba keratitis in australia: Demographics, associated factors, presentation and outcomes: A 15-year case review. Eye (Lond.) 2019, 34, 725–732. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Martin-Navarro, C.M.; Lopez-Arencibia, A.; Arnalich-Montiel, F.; Pinero, J.E.; Valladares, B. Acanthamoeba keratitis: An emerging disease gathering importance worldwide? Trends Parasitol. 2013, 29, 181–187. [Google Scholar] [CrossRef]
- Randag, A.C.; van Rooij, J.; van Goor, A.T.; Verkerk, S.; Wisse, R.P.L.; Saelens, I.E.Y.; Stoutenbeek, R.; van Dooren, B.T.H.; Cheng, Y.Y.Y.; Eggink, C.A. The rising incidence of Acanthamoeba keratitis: A 7-year nationwide survey and clinical assessment of risk factors and functional outcomes. PLoS ONE 2019, 14, e0222092. [Google Scholar] [CrossRef]
- Debnath, A.; Tunac, J.B.; Silva-Olivares, A.; Galindo-Gomez, S.; Shibayama, M.; McKerrow, J.H. In vitro efficacy of corifungin against Acanthamoeba castellanii trophozoites and cysts. Antimicrob. Agents Chemother. 2014, 58, 1523–1528. [Google Scholar] [CrossRef] [Green Version]
- Visvesvara, G.S.; Moura, H.; Schuster, F.L. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., balamuthia mandrillaris, naegleria fowleri, and sappinia diploidea. FEMS Immunol. Med. Microbiol. 2007, 50, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.C.; Shih, M.H.; Chang, K.F.; Huang, J.M.; Shin, J.W.; Lin, W.C. Characterizing clinical isolates of Acanthamoeba castellanii with high resistance to polyhexamethylene biguanide in taiwan. J. Microbiol. Immunol. Infect. 2017, 50, 570–577. [Google Scholar] [CrossRef] [Green Version]
- Walochnik, J.; Aichelburg, A.; Assadian, O.; Steuer, A.; Visvesvara, G.; Vetter, N.; Aspock, H. Granulomatous amoebic encephalitis caused by Acanthamoeba amoebae of genotype t2 in a human immunodeficiency virus-negative patient. J. Clin. Microbiol. 2008, 46, 338–340. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.A.; Yusof, M.S.; Amin, N.M. Anti-amoebic properties of carbonyl thiourea derivatives. Molecules 2014, 19, 5191–5204. [Google Scholar] [CrossRef] [Green Version]
- Perrine, D.; Chenu, J.P.; Georges, P.; Lancelot, J.C.; Saturnino, C.; Robba, M. Amoebicidal efficiencies of various diamidines against two strains of Acanthamoeba polyphaga. Antimicrob. Agents Chemother. 1995, 39, 339–342. [Google Scholar] [CrossRef] [Green Version]
- Khunkitti, W.; Lloyd, D.; Furr, J.R.; Russell, A.D. Aspects of the mechanisms of action of biguanides on trophozoites and cysts of Acanthamoeba castellanii. J. Appl. Microbiol. 1997, 82, 107–114. [Google Scholar] [CrossRef]
- Nakisah, M.A.; Ida Muryany, M.Y.; Fatimah, H.; Nor Fadilah, R.; Zalilawati, M.R.; Khamsah, S.; Habsah, M. Anti-amoebic properties of a malaysian marine sponge aaptos sp. On Acanthamoeba castellanii. World J. Microbiol. Biotechnol. 2012, 28, 1237–1244. [Google Scholar] [CrossRef]
- Mogoa, E.; Bodet, C.; Legube, B.; Hechard, Y. Acanthamoeba castellanii: Cellular changes induced by chlorination. Exp. Parasitol. 2010, 126, 97–102. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Grinstaff, M.W. Biomedical applications of dendrimers: A tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar] [CrossRef]
- Garcia-Gallego, S.; Franci, G.; Falanga, A.; Gomez, R.; Folliero, V.; Galdiero, S.; de la Mata, F.J.; Galdiero, M. Function oriented molecular design: Dendrimers as novel antimicrobials. Molecules 2017, 22, 1581. [Google Scholar] [CrossRef]
- Heredero-Bermejo, I.; Sanchez-Nieves, J.; Soliveri, J.; Gomez, R.; de la Mata, F.J.; Copa-Patino, J.L.; Perez-Serrano, J. In vitro anti-Acanthamoeba synergistic effect of chlorhexidine and cationic carbosilane dendrimers against both trophozoite and cyst forms. Int. J. Pharm. 2016, 509, 1–7. [Google Scholar] [CrossRef]
- Heredero-Bermejo, I.; Hernández-Ros, J.M.; Sánchez-García, L.; Maly, M.; Verdú-Expósito, C.; Soliveri, J.; Javier de la Mata, F.; Copa-Patiño, J.L.; Pérez-Serrano, J.; Sánchez-Nieves, J.; et al. Ammonium and guanidine carbosilane dendrimers and dendrons as microbicides. Eur. Polym. J. 2018, 101, 159–168. [Google Scholar] [CrossRef]
- Martin-Perez, T.; Lozano-Cruz, T.; Criado-Fornelio, A.; Ortega, P.; Gomez, R.; de la Mata, F.J.; Perez-Serrano, J. Synthesis and in vitro activity of new biguanide-containing dendrimers on pathogenic isolates of Acanthamoeba polyphaga and Acanthamoeba griffini. Parasitol. Res. 2019, 118, 1953–1961. [Google Scholar] [CrossRef]
- Fuentes-Paniagua, E.; Hernández-Ros, J.M.; Sánchez-Milla, M.; Camero, M.; Malý, M.; Pérez-Serrano, J.; Copa-Patiño, J.L.; Sánchez-Nieves, J.; Soliveri, J.; Gomez, R.; et al. Carbosilane cationic dendrimers synthesized by thiol–ene click chemistry and their use as antibacterial agents. RSC Adv. 2014, 3, 1256–1265. [Google Scholar] [CrossRef]
- Fuentes-Paniagua, E.; Sánchez-Nieves, J.; Hernández-Ros, J.M.; Fernández-Ezequiel, A.; Soliveri, J.; Copa-Patiño, J.L.; Gómez, R.; Javier de la Mata, F. Structure–activity relationship study of cationic carbosilane dendritic systems as antibacterial agents. RSC Adv. 2016, 6, 7022–7033. [Google Scholar] [CrossRef]
- Heredero-Bermejo, I.; San Juan Martin, C.; Soliveri de Carranza, J.; Copa-Patino, J.L.; Perez-Serrano, J. Acanthamoeba castellanii: In vitro uah-t17c3 trophozoite growth study in different culture media. Parasitol. Res. 2012, 110, 2563–2567. [Google Scholar] [CrossRef]
- Heredero-Bermejo, I.; Copa-Patino, J.L.; Soliveri, J.; Fuentes-Paniagua, E.; de la Mata, F.J.; Gomez, R.; Perez-Serrano, J. Evaluation of the activity of new cationic carbosilane dendrimers on trophozoites and cysts of Acanthamoeba polyphaga. Parasitol. Res. 2015, 114, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Ortega, P.; Macarena Cobaleda, B.; Hernandez-Ros, J.M.; Fuentes-Paniagua, E.; Sanchez-Nieves, J.; Tarazona, M.P.; Copa-Patino, J.L.; Soliveri, J.; de la Mata, F.J.; Gomez, R. Hyperbranched polymers versus dendrimers containing a carbosilane framework and terminal ammonium groups as antimicrobial agents. Org. Biomol. Chem. 2011, 9, 5238–5248. [Google Scholar] [CrossRef] [PubMed]
- Seal, D. Treatment of Acanthamoeba keratitis. Expert Rev. Anti Infect. Ther. 2003, 1, 205–208. [Google Scholar] [CrossRef]
- Lim, N.; Goh, D.; Bunce, C.; Xing, W.; Fraenkel, G.; Poole, T.R.; Ficker, L. Comparison of polyhexamethylene biguanide and chlorhexidine as monotherapy agents in the treatment of Acanthamoeba keratitis. Am. J. Ophthalmol. 2008, 145, 130–135. [Google Scholar] [CrossRef]
- Wysenbeek, Y.S.; Blank-Porat, D.; Harizman, N.; Wygnanski-Jaffe, T.; Keller, N.; Avni, I. The reculture technique: Individualizing the treatment of Acanthamoeba keratitis. Cornea 2000, 19, 464–467. [Google Scholar] [CrossRef]
- Kumar, R.; Lloyd, D. Recent advances in the treatment of Acanthamoeba keratitis. Clin. Infect. Dis. 2002, 35, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Z.; Beck-Tan, N.C.; Dhurjati, P.; van Dyk, T.K.; LaRossa, R.A.; Cooper, S.L. Quaternary ammonium functionalized poly(propylene imine) dendrimers as effective antimicrobials: Structure-activity studies. Biomacromolecules 2000, 1, 473–480. [Google Scholar] [CrossRef]
- Llamazares, C.; Sanz Del Olmo, N.; Ortega, P.; Gomez, R.; Soliveri, J.; de la Mata, F.J.; Garcia-Gallego, S.; Copa-Patino, J.L. Antibacterial effect of carbosilane metallodendrimers in planktonic cells of gram-positive and gram-negative bacteria and staphylococcus aureus biofilm. Biomolecules 2019, 9, 405. [Google Scholar] [CrossRef] [Green Version]
- Mintzer, M.A.; Dane, E.L.; O’Toole, G.A.; Grinstaff, M.W. Exploiting dendrimer multivalency to combat emerging and re-emerging infectious diseases. Mol. Pharm. 2012, 9, 342–354. [Google Scholar] [CrossRef] [Green Version]
- Wronska, N.; Majoral, J.P.; Appelhans, D.; Bryszewska, M.; Lisowska, K. Synergistic effects of anionic/cationic dendrimers and levofloxacin on antibacterial activities. Molecules 2019, 24, 2894. [Google Scholar] [CrossRef] [Green Version]
- Mhlwatika, Z.; Aderibigbe, B.A. Application of dendrimers for the treatment of infectious diseases. Molecules 2018, 23, 2205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, E.L.; Morilla, M.J. Nanotechnological approaches against chagas disease. Adv. Drug Deliv. Rev. 2010, 62, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Weber, N.; Ortega, P.; Clemente, M.I.; Shcharbin, D.; Bryszewska, M.; de la Mata, F.J.; Gomez, R.; Munoz-Fernandez, M.A. Characterization of carbosilane dendrimers as effective carriers of sirna to hiv-infected lymphocytes. J. Control. Release 2008, 132, 55–64. [Google Scholar] [CrossRef]
- de Las Cuevas, N.; Garcia-Gallego, S.; Rasines, B.; de la Mata, F.J.; Guijarro, L.G.; Munoz-Fernandez, M.A.; Gomez, R. In vitro studies of water-stable cationic carbosilane dendrimers as delivery vehicles for gene therapy against hiv and hepatocarcinoma. Curr. Med. Chem. 2012, 19, 5052–5061. [Google Scholar] [CrossRef] [PubMed]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasines, B.; Hernandez-Ros, J.M.; de las Cuevas, N.; Copa-Patino, J.L.; Soliveri, J.; Munoz-Fernandez, M.A.; Gomez, R.; de la Mata, F.J. Water-stable ammonium-terminated carbosilane dendrimers as efficient antibacterial agents. Dalton Trans. 2009, 40, 8704–8713. [Google Scholar] [CrossRef]
- Martinez, A.; Rojas, N.; Garcia, L.; Gonzalez, F.; Dominguez, M.; Catalan, A. In vitro activity of terpenes against candida albicans and ultrastructural alterations. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 553–559. [Google Scholar] [CrossRef]
- Fatimah Hashim, N.m. Visualization on the effect of chlorhexidine gluconate, a biocide on Acanthamoeba sp by electron microscopy. Malays. J. Microsc. 2013, 9, 154–159. [Google Scholar]
- Khunkitti, W.; Hann, A.C.; Lloyd, D.; Furr, J.R.; Russell, A.D. Biguanide-induced changes in Acanthamoeba castellanii: An electron microscopic study. J. Appl. Microbiol. 1998, 84, 53–62. [Google Scholar] [CrossRef]
- Khunkitti, W.; Lloyd, D.; Furr, J.R.; Russell, A.D. Acanthamoeba castellanii: Growth, encystment, excystment and biocide susceptibility. J. Infect. 1998, 36, 43–48. [Google Scholar] [CrossRef]
- Seal, D.V. Acanthamoeba keratitis update-incidence, molecular epidemiology and new drugs for treatment. Eye (Lond.) 2003, 17, 893–905. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Z.; Cooper, S.L. Interactions between dendrimer biocides and bacterial membranes. Biomaterials 2002, 23, 3359–3368. [Google Scholar] [CrossRef]









| Compound | R | Core | N | Functional Group | IC50 (mg/L) | MCC (mg/L) |
|---|---|---|---|---|---|---|
| [G1O3(S-NMe3)]6+ | 1 | P | 6 | -NMe3+ | 16.9 + 0.8 | >512 |
| [G1Si(S-NMe3)]4+ | 2 | S | 4 | -NMe3+ | 430.1 + 5.8 | >512 |
| [G2Si(S-NMe3)]8+ | 3 | S | 8 | -NMe3+ | 46.5 + 0.9 | >512 |
| [G1O3(S-NH3)6]6+ | 4 | P | 6 | -NH3+ | 2.4 + 0.1 | 256 |
| [G1Si(NMe3)]4+ | 5 | S | 4 | -NMe3+ | 7.8 + 0.2 | 512 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heredero-Bermejo, I.; Martín-Pérez, T.; Copa-Patiño, J.L.; Gómez, R.; de la Mata, F.J.; Soliveri, J.; Pérez-Serrano, J. Ultrastructural Study of Acanthamoeba polyphaga Trophozoites and Cysts Treated In Vitro with Cationic Carbosilane Dendrimers. Pharmaceutics 2020, 12, 565. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12060565
Heredero-Bermejo I, Martín-Pérez T, Copa-Patiño JL, Gómez R, de la Mata FJ, Soliveri J, Pérez-Serrano J. Ultrastructural Study of Acanthamoeba polyphaga Trophozoites and Cysts Treated In Vitro with Cationic Carbosilane Dendrimers. Pharmaceutics. 2020; 12(6):565. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12060565
Chicago/Turabian StyleHeredero-Bermejo, Irene, Tania Martín-Pérez, José Luis Copa-Patiño, Rafael Gómez, Francisco Javier de la Mata, Juan Soliveri, and Jorge Pérez-Serrano. 2020. "Ultrastructural Study of Acanthamoeba polyphaga Trophozoites and Cysts Treated In Vitro with Cationic Carbosilane Dendrimers" Pharmaceutics 12, no. 6: 565. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12060565