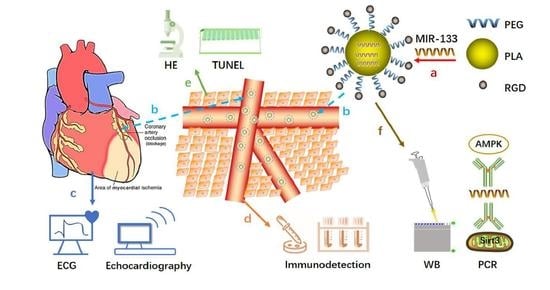
RGD-PEG-PLA Delivers MiR-133 to Infarct Lesions of Acute Myocardial Infarction Model Rats for Cardiac Protection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Preparation and Characterization of Nanoparticles
2.4. Establishment of AMI Model in Rats and Administration
2.5. In Vivo Biodistribution Evaluation
2.6. Assessment of Heart Functions
2.6.1. Electrocardiogram
2.6.2. Echocardiography
2.7. Biochemical Indicators Detection
2.7.1. Expression of Cardiac-Specific Markers
2.7.2. Assessment of Oxidative Stress Markers
2.7.3. Assessment of Inflammatory Cytokines
2.7.4. Assessment of Endothelial Active Substances
2.8. Histopathological Examination
2.9. Detection of Apoptosis by the TUNEL Assay
2.10. Immunohistochemical Staining
2.11. Real-Time Polymerase Chain Reaction (PCR) Analysis
2.12. Western Blotting Analysis
2.13. Statistical Analysis
3. Results
3.1. Characterization of Nanoparticles
3.2. Biodistribution of RGD-PEG-PLA Nanoparticles In Vivo
3.3. Effects of RGD-PEG-PLA/miR-133 on the Electrocardiogram of AMI Rats
3.4. Effects of RGD-PEG-PLA/miR-133 on the Echocardiography of AMI Rats
3.4.1. Effects of RGD-PEG-PLA/miR-133 on the Left Ventricle Morphology in AMI Rats
3.4.2. Effects of RGD-PEG-PLA/miR-133 on the Left Ventricular Function in AMI Rats
3.5. Effects of RGD-PEG-PLA/miR-133 on the Biochemical Indicators of AMI Rats
3.5.1. Effects of RGD-PEG-PLA/miR-133 on Cardiac-Specific Markers
3.5.2. Effects of RGD-PEG-PLA/miR-133 on Oxidative Stress Markers
3.5.3. Effects of RGD-PEG-PLA/miR-133 on Serum Inflammatory Cytokines
3.5.4. Effects of RGD-PEG-PLA/miR-133 on Endothelial Active Substances
3.6. Effects of RGD-PEG-PLA/miR-133 on the Histopathological Histology of AMI Rats
3.7. Effects of RGD-PEG-PLA/miR-133 on Cardiomyocyte Apoptosis in AMI Rats
3.8. Effects of RGD-PEG-PLA/miR-133 on the Expression of the SIRT3/AMPK Pathway in AMI Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nicolini, G.; Forini, F.; Kusmic, C.; Pitto, L.; Mariani, L.; Iervasi, G. Early and short-term triiodothyronine supplementation prevents adverse postischemic cardiac remodeling: Role of transforming growth factor-beta 1 and antifibrotic mirna signaling. Mol. Med. 2015, 21, 900–911. [Google Scholar] [CrossRef]
- Pisano, F.; Altomare, C.; Cervio, E.; Barile, L.; Rocchetti, M.; Ciuffreda, M.C.; Malpasso, G.; Copes, F.; Mura, M.; Danieli, P.; et al. Combination of miRNA499 and miRNA133 exerts a synergic effect on cardiac differentiation. Stem Cells 2015, 33, 1187–1199. [Google Scholar] [CrossRef]
- Zhang, X.G.; Wang, L.Q.; Guan, H.L. Investigating the expression of miRNA-133 in animal models of myocardial infarction and its effect on cardiac function. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5934–5940. [Google Scholar] [PubMed]
- Yu, Y.; Liu, H.; Yang, D.; He, F.; Yuan, Y.; Guo, J.; Hu, J.; Yu, J.; Yan, X.; Wang, S.; et al. Aloe-emodin attenuates myocardial infarction and apoptosis via up-regulating miR-133 expression. Pharmacol. Res. 2019, 146, 104315. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, C.; Zhang, J.; Jiao, Z.; Dong, N.; Wang, G.; Wang, Z.; Wang, L. Localized injection of miRNA-21-enriched extracellular vesicles effectively restores cardiac function after myocardial infarction. Theranostics 2019, 9, 2346–2360. [Google Scholar] [CrossRef] [PubMed]
- Bejerano, T.; Etzion, S.; Elyagon, S.; Etzion, Y.; Cohen, S. Nanoparticle delivery of miRNA-21 mimic to cardiac macrophages improves myocardial remodeling after myocardial infarction. Nano Lett. 2018, 18, 5885–5891. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiang, J.; Wang, Y.; Jia, Q.; Dai, S.; Wang, Y.; Lv, L.; Wang, J. rLj-RGD3, a novel recombinant toxin protein from Lampetra japonica, prevents coronary thrombosis-induced acute myocardial infarction by inhibiting platelet functions in rats. Biochem. Biophys. Res. Commun. 2018, 498, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Goldbergova, M.P.; Lipkova, J.; Fedorko, J.; Sevcikova, J.; Parenica, J.; Spinar, J.; Masarik, M.; Vasku, A. MicroRNAs in pathophysiology of acute myocardial infarction and cardiogenic shock. Bratisl. Lek. Listy 2018, 119, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Xiao, F.-Y.; Shan, P.-R.; Su, L.; Chen, D.-L.; Ding, J.-Y.; Wang, Z.-Q. Overexpression of microRNA-133a inhibits ischemia-reperfusion-induced cardiomyocyte apoptosis by targeting DAPK2. J. Hum. Genet. 2015, 60, 709–716. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.-T.; Yu, N.; Wang, Y.; Zhang, H.; Wan, K.; Sun, X.; Zhang, C.-S. Role of miR-133a in regulating TGF-β1 signaling pathway in myocardial fibrosis after acute myocardial infarction in rats. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8588–8597. [Google Scholar]
- Horie, T.; Ono, K.; Nishi, H.; Iwanaga, Y.; Nagao, K.; Kinoshita, M.; Kuwabara, Y.; Takanabe, R.; Hasegawa, K.; Kita, T.; et al. MicroRNA-133 regulates the expression of GLUT4 by targeting KLF15 and is involved in metabolic control in cardiac myocytes. Biochem. Biophys. Res. Commun. 2009, 389, 315–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Zhao, Y.; Chen, W.; Xie, L.; Zhao, Z.-A.; Yang, J.; Chen, Y.; Lei, W.; Shen, Z. MicroRNA-133 overexpression promotes the therapeutic efficacy of mesenchymal stem cells on acute myocardial infarction. Stem Cell Res. Ther. 2017, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Yao, Y.; Wang, T.; Huang, H.; Xia, H. Tanshinone IIA ameliorates apoptosis of myocardiocytes by up-regulation of miR-133 and suppression of Caspase-9. Eur. J. Pharmacol. 2017, 815, 343–350. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Xiao, J.; Ren, A.-J.; Zhang, Y.-F.; Zhang, H.; Chen, M.; Xie, B.; Gao, X.-G.; Wang, Y.-W. Role of miR-1 and miR-133a in myocardial ischemic postconditioning. J. Biomed. Sci. 2011, 18, 22. [Google Scholar] [CrossRef] [Green Version]
- Bostjancic, E.; Zidar, N.; Stajer, D.; Glavac, D. MicroRNAs, miR-1, miR-133a/b and miR-208 in infarcted and remote myocardium of human myocardial infarction with the focus on the ventricular fibrilation and/or tachycardia. FEBS J. 2012, 279, 312. [Google Scholar]
- Xu, C.; Hu, Y.; Hou, L.; Ju, J.; Li, X.; Du, N.; Guan, X.; Liu, Z.; Zhang, T.; Qin, W.; et al. β-Blocker carvedilol protects cardiomyocytes against oxidative stress-induced apoptosis by up-regulating miR-133 expression. J. Mol. Cell. Cardiol. 2014, 75, 111–121. [Google Scholar] [CrossRef]
- Muniyandi, P.; Palaninathan, V.; Mizuki, T.; Maekawa, T.; Hanajiri, T.; Mohamed, M.S. Poly(lactic-co-glycolic acid)/polyethylenimine nanocarriers for direct genetic reprogramming of microRNA targeting cardiac fibroblasts. ACS Appl. Nano Mater. 2020, 3, 2491–2505. [Google Scholar] [CrossRef]
- Ma, D.; Liu, H.; Zhao, P.; Ye, L.; Zou, H.; Zhao, X.; Dai, H.; Kong, X.; Liu, P. Programing assembling/releasing multifunctional miRNA nanomedicine to treat prostate cancer. ACS Appl. Mater. Interfaces 2020, 12, 9032–9040. [Google Scholar] [CrossRef]
- Cai, X.X.; Zhu, Q.X.; Zeng, Y.; Zeng, Q.; Chen, X.L.; Zhan, Y.H. Manganese oxide nanoparticles as MRI contrast agents in tumor multimodal imaging and therapy. Int. J. Nanomed. 2019, 14, 8321–8344. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.P.A.; Talman, V.; Torrieri, G.; Liu, D.; Marques, G.; Moslova, K.; Liu, Z.; Pinto, J.F.; Hirvonen, J.; Ruskoaho, H.; et al. Dual-drug delivery using dextran-functionalized nanoparticles targeting cardiac fibroblasts for cellular reprogramming. Adv. Funct. Mater. 2018, 28, 1705134. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Pan, J. Baicalin-loaded PEGylated lipid nanoparticles: Characterization, pharmacokinetics, and protective effects on acute myocardial ischemia in rats. Drug Deliv. 2016, 23, 3696–3703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duro-Castano, A.; Gallon, E.; Decker, C.; Vicent, M.J. Modulating angiogenesis with integrin-targeted nanomedicines. Adv. Drug Deliv. Rev. 2017, 119, 101–119. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Zhang, Y.; Yang, S.; Wang, J.; Zhang, X.; Zhang, Q. RGD-modified polymeric micelles as potential carriers for targeted delivery to integrin-overexpressing tumor vasculature and tumor cells. J. Drug Target. 2009, 17, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, C.; Dou, J.; Xi, Y.; Lou, H.; Zhai, G. Development of RGD-functionalized PEG-PLA micelles for delivery of curcumin. J. Biomed. Nanotechnol. 2015, 11, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Sun, B.; Yang, L.; Ma, C.; Li, S. RVG29-modified microRNA-loaded nanoparticles improve ischemic brain injury by nasal delivery. Drug Deliv. 2020, 27, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhou, J.; Yang, B.; Yu, Y. MicroRNA-340-5p inhibits hypoxia/reoxygenation-induced apoptosis and oxidative stress in cardiomyocytes by regulating the Act1/NF-κB pathway. J. Cell. Biochem. 2019, 120, 14618–14627. [Google Scholar] [CrossRef]
- Geng, X.-Y.; Xiao, N.; Han, Y.; Li, Y.-J. Platelet microparticles: A tool to predict infarction area in rats. J. Investig. Surg. 2019, 1–6. [Google Scholar] [CrossRef]
- Jin, H.; Li, D.Y.; Chernogubova, E.; Sun, C.; Busch, A.; Eken, S.M.; Saliba-Gustafsson, P.; Winter, H.; Winski, G.; Raaz, U.; et al. Local delivery of miR-21 stabilizes fibrous caps in vulnerable atherosclerotic lesions. Mol. Ther. 2018, 26, 1040–1055. [Google Scholar] [CrossRef] [Green Version]
- Cook, R.L.; Householder, K.T.; Chung, E.P.; Prakapenka, A.V.; DiPerna, D.M.; Sirianni, R.W. A critical evaluation of drug delivery from ligand modified nanoparticles: Confounding small molecule distribution and efficacy in the central nervous system. J. Control. Release 2015, 220, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Menown, I.B.A.; Mackenzie, G.; Adgey, A.A.J. Optimizing the initial 12-lead electrocardiographic diagnosis of acute myocardial infarction. Eur. Heart J. 2000, 21, 275–283. [Google Scholar] [CrossRef]
- Koentges, C.; Bode, C.; Bugger, H. SIRT3 in cardiac physiology and disease. Front. Cardiovasc. Med. 2016, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchelson, K.R.; Qin, W.-Y. Roles of the canonical myomiRs mi R-1,-133 and-206 in cell development and disease. World J. Biol. Chem. 2015, 6, 162–208. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xue, R.-Q.; Lu, Y.; Yong, S.-Y.; Wu, Q.; Cui, Y.-L.; Zuo, X.-T.; Yu, X.-J.; Zhao, M.; Zang, W.-J. Choline ameliorates cardiac hypertrophy by regulating metabolic remodelling and UPRmt through SIRT3-AMPK pathway. Cardiovasc. Res. 2018, 115, 530–545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, Y.; Li, Y.; Xu, C.; Li, X.; Zhu, D.; Zhang, Y.; Xing, S.; Wang, H.; Zhang, Z.; et al. Tanshinone IIA improves miR-133 expression through MAPK ERK1/2 pathway in hypoxic cardiac myocytes. Cell. Physiol. Biochem. 2012, 30, 843–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Li, X.; Zhou, Y.; Shi, H.; Xu, C.; He, H.; Wang, S.; Xiong, X.; Zhang, Y.; Du, Z.; et al. Downregulation of miR-133 via MAPK/ERK signaling pathway involved in nicotine-induced cardiomyocyte apoptosis. Naunyn. Schmiedebergs. Arch. Pharmacol. 2014, 387, 197–206. [Google Scholar] [CrossRef]
- Yu, C.; Xue, J.; Zhu, W.; Jiao, Y.; Zhang, S.; Cao, J. Warburg meets non-coding RNAs: The emerging role of ncRNA in regulating the glucose metabolism of cancer cells. Tumor Biol. 2015, 36, 81–94. [Google Scholar] [CrossRef]
- Klishadi, M.S.; Zarei, F.; Hejazian, S.H.; Moradi, A. Losartan protects the heart against ischemia reperfusion injury: Sirtuin3 involvement. J. Pharm. Pharm. Sci. 2015, 18. [Google Scholar] [CrossRef]
- Bheri, S.; Davis, M.E. Nanoparticle-hydrogel system for post-myocardial infarction delivery of microRNA. ACS Nano 2019, 13, 9702–9706. [Google Scholar] [CrossRef]
- Curaj, A.; Simsekyilmaz, S.; Staudt, M.; Liehn, E. Minimal invasive surgical procedure of inducing myocardial infarction in mice. J. Vis. Exp. 2015, e52197. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Jing, Q. Non-coding RNAs as biomarkers for acute myocardial infarction. Acta Pharmacol. Sin. 2018, 39, 1110–1119. [Google Scholar] [CrossRef]
- Quagliariello, V.; Coppola, C.; Mita, D.G.; Piscopo, G.; Iaffaioli, R.V.; Botti, G.; Maurea, N. Low doses of Bisphenol A have pro-inflammatory and pro-oxidant effects, stimulate lipid peroxidation and increase the cardiotoxicity of Doxorubicin in cardiomyoblasts. Environ. Toxicol. Pharmacol. 2019, 69, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.H.; Shim, M.K.; Kim, G.L.; Kim, S.; Kang, H.; Kim, J.-H. Hypoxia-responsive folic acid conjugated glycol chitosan nanoparticle for enhanced tumor targeting treatment. Int. J. Pharm. 2020, 580, 119237. [Google Scholar] [CrossRef] [PubMed]





















© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, B.; Liu, S.; Hao, R.; Dong, X.; Fu, L.; Han, B. RGD-PEG-PLA Delivers MiR-133 to Infarct Lesions of Acute Myocardial Infarction Model Rats for Cardiac Protection. Pharmaceutics 2020, 12, 575. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12060575
Sun B, Liu S, Hao R, Dong X, Fu L, Han B. RGD-PEG-PLA Delivers MiR-133 to Infarct Lesions of Acute Myocardial Infarction Model Rats for Cardiac Protection. Pharmaceutics. 2020; 12(6):575. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12060575
Chicago/Turabian StyleSun, Bixi, Shuwen Liu, Rubin Hao, Xinyue Dong, Lanbo Fu, and Bing Han. 2020. "RGD-PEG-PLA Delivers MiR-133 to Infarct Lesions of Acute Myocardial Infarction Model Rats for Cardiac Protection" Pharmaceutics 12, no. 6: 575. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12060575