Manipulation of Medicinal Products for Oral Administration to Paediatric Patients at a German University Hospital: An Observational Study
Abstract
:1. Introduction
2. Methods
2.1. Design
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection
2.4. Ethical Approval
2.5. Definitions
2.6. Data Analysis
3. Results
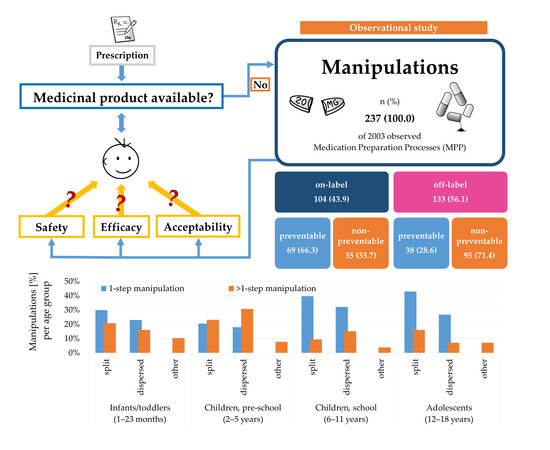
3.1. Prevalence of Manipulations
3.2. Frequency of Manipulations
3.3. Licensing Status and Preventability of Manipulations
3.4. Active Substances and Therapeutic Subgroups Affected by Manipulations
3.5. Dosage Forms Manipulated and Type of Manipulation
3.5.1. Manipulation Type Classified by Age Group
3.5.2. Proportion of the Dosage Form Administered
3.6. Root Causes for Manipulation
4. Discussion
4.1. Frequency of Manipulations
4.2. Licensing Status and Preventability of Manipulations
4.3. Active Substances and Therapeutic Subgroups Affected by Manipulation
4.4. Dosage Forms and Type of Manipulation
4.5. Proportion of the Dosage Form Administered
4.6. Root Cause for Manipulation
4.7. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
| AE | Adverse Event |
| ASS | Acetylsalicylic acid |
| CHMP | Committee on Medicinal Products for Human Use |
| IU | International Units |
| Man. | Manipulation |
| MPP | Medication preparation processes |
| MUPS | Multiple Pellets Unit System |
| NTIs | Drugs with Narrow Therapeutic Index |
| PDCO | Paediatric Committee |
| PUMA | Paediatric Use Marketing Authorization |
| SmPC | Summary of Product Characteristics |
References
- Van Riet-Nales, D.A.; Schobben, A.F.A.M.; Vromans, H.; Egberts, T.; A Rademaker, C.M. Safe and effective pharmacotherapy in infants and preschool children: Importance of formulation aspects. Arch. Dis. Child. 2016, 101, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, S.; Neubert, A.; Rascher, W. The Safety of Drug Therapy in Children. Dtsch. Aerzteblatt Online 2015, 112, 781–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Standing, J.F.; Tuleu, C. Paediatric formulations—Getting to the heart of the problem. Int. J. Pharm. 2005, 300, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Kearns, G.L.; Abdel-Rahman, S.M.; Alander, S.W.; Blowey, D.L.; Leeder, J.S.; Kauffman, R.E. Developmental Pharmacology—Drug Disposition, Action, and Therapy in Infants and Children. N. Engl. J. Med. 2003, 349, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Breitkreutz, J.; Boos, J.; Breitkreutz, J. Paediatric and geriatric drug delivery. Expert Opin. Drug Deliv. 2006, 4, 37–45. [Google Scholar] [CrossRef]
- Lohmann, K.; Ferber, J.; Haefeli, W.E.; Störzinger, D.; Schwald, M.; Seidling, H.M.; Haefeli, W.E. Knowledge and training needs of nurses and physicians on unsuitable drugs for patients with dysphagia or feeding tubes. J. Clin. Nurs. 2015, 24, 3016–3019. [Google Scholar] [CrossRef]
- O’Brien, F.; Clapham, D.; Krysiak, K.; Batchelor, H.; Field, P.; Caivano, G.; Pertile, M.; Nunn, A.; Tuleu, C. Making Medicines Baby Size: The Challenges in Bridging the Formulation Gap in Neonatal Medicine. Int. J. Mol. Sci. 2019, 20, 2688. [Google Scholar] [CrossRef] [Green Version]
- Richey, R.H.; Shah, U.; Peak, M.; Craig, J.; Ford, J.L.; Barker, C.E.; Nunn, A.; Turner, M. Manipulation of drugs to achieve the required dose is intrinsic to paediatric practice but is not supported by guidelines or evidence. BMC Pediatr. 2013, 13, 81. [Google Scholar] [CrossRef] [Green Version]
- Bjerknes, K.; Bøyum, S.; Kristensen, S.; Brustugun, J.; Wang, S. Manipulating tablets and capsules given to hospitalised children in Norway is common practice. Acta Paediatr. 2017, 106, 503–508. [Google Scholar] [CrossRef]
- Fontan, J.-E.; Mille, F.; Brion, F. L’administration des médicaments à l’enfant hospitalisé. Arch. Pédiatrie 2004, 11, 1173–1184. [Google Scholar] [CrossRef]
- Nunn, A.; Richey, R.; Shah, U.; Barker, C.; Craig, J.; Peak, M.; Ford, J.; Turner, M. Estimating the requirement for manipulation of medicines to provide accurate doses for children. Eur. J. Hosp. Pharm. 2012, 20, 3–7. [Google Scholar] [CrossRef]
- Jacques, E.R.; Alexandridis, P. Tablet Scoring: Current Practice, Fundamentals, and Knowledge Gaps. Appl. Sci. 2019, 9, 3066. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency. Reflection Paper: Formulations of Choice for the Paediatric Population; (EMEA/CHMP/PEG/194810/2005); European Medicines Agency: London, UK, 2006. [Google Scholar]
- Richey, R.; Hughes, C.; Craig, J.; Shah, U.U.; Ford, J.L.; Barker, C.E.; Peak, M.; Nunn, A.; Turner, M. A systematic review of the use of dosage form manipulation to obtain required doses to inform use of manipulation in paediatric practice. Int. J. Pharm. 2017, 518, 155–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornish, P. “Avoid the crush”: Hazards of medication administration in patients with dysphagia or a feeding tube. Can. Med. Assoc. J. 2005, 172, 871–872. [Google Scholar] [CrossRef] [Green Version]
- Paparella, S. Identified Safety Risks With Splitting and Crushing Oral Medications. J. Emerg. Nurs. 2010, 36, 156–158. [Google Scholar] [CrossRef]
- Lohmann, K.; Ferber, J.; Send, A.F.J.; Haefeli, W.E.; Seidling, H.M. Inappropriate crushing information on ward lists: Cytotoxic drugs, capsules, and modified release formulations are gravely neglected. Eur. J. Clin. Pharmacol. 2014, 70, 565–573. [Google Scholar] [CrossRef]
- Best, B.M.; Capparelli, E.V.; Diep, H.; Rossi, S.S.; Farrell, M.J.; Williams, E.; Lee, G.; Anker, J.N.V.D.; Rakhmanina, N. Pharmacokinetics of lopinavir/ritonavir crushed versus whole tablets in children. JAIDS J. Acquir. Immune Defic. Syndr. 2011, 58, 385–391. [Google Scholar] [CrossRef] [Green Version]
- Brustugun, J.; Notaker, N.; Paetz, L.H.; Tho, I.; Bjerknes, K. Adjusting the dose in paediatric care: Dispersing four different aspirin tablets and taking a proportion. Eur. J. Hosp. Pharm. 2019, 10, 1136. [Google Scholar] [CrossRef] [Green Version]
- Brustugun, J.; Notaker, N.; Paetz, L.H.; Tho, I.; Bjerknes, K. Adjusting the dose in paediatric care by dispersing fragments of four different aspirin tablets. Acta Paediatr. 2020. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, J.M.; Nathan, J.P.; Plakogiannis, F. Weight Variability of Pharmacist-Dispensed Split Tablets. J. Am. Pharm. Assoc. 2002, 42, 200–205. [Google Scholar] [CrossRef]
- Van der Vossen, A.C.; Al-Hassany, L.; Buljac, S.; Brugma, J.-D.; Vulto, A.G.; Hanff, L.M. Manipulation of oral medication for children by parents and nurses occurs frequently and is often not supported by instructions. Acta Paediatr. 2019, 108, 1475–1481. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency. Guideline on Pharmaceutical Development of Medicines for Paediatric Use; (EMA/CHMP/QWP/805880/2012 Rev. 2); European Medicines Agency: London, UK, 2012. [Google Scholar]
- Richey, R.H.; Craig, J.; Shah, U.U.; Nunn, A.; Turner, M.; Barker, C.E.; Ford, J.L.; Peak, M. MODRIC—Manipulation of drugs in children. Int. J. Pharm. 2013, 457, 339–341. [Google Scholar] [CrossRef]
- WIdO Wissenschaftliches Institut der AOK. Anatomisch-therapeutisch-chemische Klassifikation mit Tagesdosen. Amtliche Fassung des ATC-Index mit DDD-Angaben für Deutschland im Jahre. 2018. Available online: http://wido.de/amtl_atc-code.html (accessed on 6 March 2018).
- European Commission. State of Paediatric Medicines in the EU—10 Years of the EU Paediatric Regulation; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Wimmer, S.; Rascher, W.; McCarthy, S.; Neubert, A. The EU Paediatric Regulation: Still a Large Discrepancy Between Therapeutic Needs and Approved Paediatric Investigation Plans. Pediatr. Drugs 2014, 16, 397–406. [Google Scholar] [CrossRef]
- Kuchenbuch, M.; Chemaly, N.; Henniene, K.M.; Kaminska, A.; Chiron, C.; Nabbout, R. Off-label use and manipulations of antiepileptic drugs in children: Analysis of the outpatient prescriptions in a tertiary center. Epilepsy Behav. E B 2018, 82, 133–139. [Google Scholar] [CrossRef]
- Johannessen Landmark, C.; Johannessen, S.I.; Patsalos, P.N. Therapeutic drug monitoring of antiepileptic drugs: Current status and future prospects. Expert Opin. Drug Metab. Toxicol. 2020, 1–12. [Google Scholar] [CrossRef]
- Deutsche Gesellschaft für Kinderendokrinologie und–Diabetologie (DGKED) e.V. S1-Leitlinie Vitamin-D-Mangel Rachitis. AWMF online 2016. Available online: https://www.awmf.org/uploads/tx_szleitlinien/174-007l_S1_Vitamin-D-Mangel_Rachitis_2016-04.pdf (accessed on 29 March 2020).
- AstraZeneca GmbH. Antra MUPS® 10 mg Magensaftresistente Tabletten, Antra MUPS® 20 mg Magensaftresistente Tabletten; AstraZeneca GmbH: Wedel, Germany, 2018. [Google Scholar]
- Boussery, K.; De Smet, J.; De Cock, P.; Velde, S.V.; Mehuys, E.; De Paepe, P.; Remon, J.P.; Van Bocxlaer, J.F.P.; Van Winckel, M. Pharmacokinetics of two formulations of omeprazole administered through a gastrostomy tube in patients with severe neurodevelopmental problems. Br. J. Clin. Pharmacol. 2011, 72, 990–996. [Google Scholar] [CrossRef]
- Chua, H.M.; Richer, N.H.; Swedrowska, M.; Ingham, S.; Tomlin, S.; Forbes, B. Dissolution of Intact, Divided and Crushed Circadin Tablets: Prolonged vs. Immediate Release of Melatonin. Pharmaceutics 2016, 8, 2. [Google Scholar] [CrossRef]
- Infectopharm Arzneimittel und Consilium GmbH. Sondengängigkeit Oraler Arzneiformen von INFECTOPHARM und PÄDIA. 2019. Available online: https://infectopharm-docs.com/docs/sd-sondengaengigkeit.pdf (accessed on 22 June 2020).





| Dosage Form | Examples of Manipulations |
|---|---|
| Tablet | Splitting/cutting/crushing |
| Dispersion in liquid | |
| Administration of a proportion/segment | |
| Dispersion in liquid and withdrawing a fraction | |
| Capsule | Opening of a capsule |
| Dispersion of content in liquid | |
| Administration of a proportion of the content | |
| Dispersion in liquid and withdrawing a fraction | |
| Oral liquid | Dilution in a larger volume |
| Administration of a fraction | |
| Sachet with powder/granules | Dispersion of content in liquid and administration of a proportion |
| Administration of a proportion of the content |
| Specification | Patients Overall | Patients with Man. | Patients with Off-Label Man. | MPP | Man. | Off-Label Man. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||||||
| Overall | |||||||||||
| Total | 193 (100.0) | 110 (57.0) | 49 (25.4) | 640 (100.0) | 237 (37.0) | 133 (20.8) | |||||
| Ward | |||||||||||
| A | 88 (45.6) | 52 (59.1) | 16 (18.2) | 307 (48.0) | 114 (37.1) | 57 (18.6) | |||||
| B | 105 (54.4) | 58 (55.2) | 33 (31.4) | 333 (52.0) | 123 (36.9) | 76 (22.8) | |||||
| Gender | |||||||||||
| Female | 88 (45.6) | 48 (54.5) | 21 (23.9) | 312 (48.8) | 106 (34.0) | 59 (18.9) | |||||
| Male | 105 (54.4) | 62 (59.0) | 28 (26.7) | 328 (51.3) | 131 (39.9) | 74 (22.6) | |||||
| Age group | |||||||||||
| Newborns (0–28 days) | 2 (1.0) | 2 (100.0) | 0 (0.0) | 6 (0.9) | 2 (33.3) | 0 (0.0) | |||||
| Infants/toddlers (1–23 months) | 58 (30.1) | 42 (72.4) | 11 (19.0) | 205 (32.0) | 87 (42.4) | 40 (19.5) | |||||
| Children, pre-school (2–5 years) | 37 (19.2) | 19 (52.4) | 11 (29.7) | 111 (17.3) | 39 (35.1) | 28 (25.2) | |||||
| Children, school (6–11 years) | 36 (18.7) | 19 (52.8) | 8 (22.2) | 137 (21.4) | 53 (38.7) | 31 (22.6) | |||||
| Adolescents (12–18 years) | 60 (31.1) | 28 (46.7) | 19 (31.7) | 181 (28.3) | 56 (30.9) | 34 (18.8) | |||||
| Presence of feeding tube | 34 (17.6) | 31 (91.2) | 15 (44.1) | 230 (35.9) | 109 (47.4) | 70 (30.4) | |||||
| Patients with Man. | Patients without Man. | ||||||||||
| Mean | SD | Median | Min | Max | Mean | SD | Median | Min | Max | p-value 1 | |
| Age (years) | 6.1 | 5.8 | 3.8 | 0.1 | 17.9 | 8.0 | 5.9 | 6.3 | 0.1 | 17.8 | 0.022 |
| Weight (kg) | 22.0 | 20.8 | 12.6 | 1.3 | 120.0 | 30.8 | 22.8 | 21.0 | 1.9 | 101.0 | 0.006 |
| Duration of stay (days) | 9.9 | 13.0 | 5.0 | 1.0 | 83.0 | 5.6 | 6.1 | 3.5 | 1.0 | 74.0 | 0.005 |
| ATC Code | Therapeutic Subgroup | n | % | Active Substances (n) |
|---|---|---|---|---|
| N03 | Antiepileptics | 64 | 27 | potassium bromide (4), phenobarbital (18), topiramat (10), lamotrigine (8), vigabatrin (6), valproic acid (4), oxcarbazepine (5), lacosamide (2), brivaracetam (3), clonazepam (2), gabapentin (2) |
| A11 | Vitamins | 47 | 19.8 | cholecalciferol (46), cholecalciferol/sodium fluoride (1) |
| A02 | Drugs for acid-related disorders | 30 | 12.7 | omeprazole (27), esomeprazole (2), sodium bicarbonate (1) |
| H02 | Corticosteroids for systemic use | 14 | 5.9 | prednisolone (7), dexamethasone (4), hydrocortisone (3) |
| A12 | Mineral supplements | 8 | 3.4 | potassium/citrate (4), sodium chloride (1), potassium chloride (2), zinc (2) |
| C02 | Antihypertensives | 8 | 3.4 | clonidine (5), bosentan (1), sildenafil (2) |
| M03 | Muscle relaxants | 6 | 2.5 | baclofen (6) |
| B03 | Anti-anaemic preparations | 5 | 2.1 | ferrous gluconate (5) |
| H03 | Thyroid therapy | 5 | 2.1 | levothyroxine sodium (4), thiamazole (1) |
| L04 | Immunosuppressants | 4 | 1.7 | tacrolimus (1), everolimus (1), azathioprine (2) |
| N05 | Psycholeptics | 4 | 1.7 | melatonin (1), clobazam (2), nitrazepam (1) |
| C07 | Beta blocking agents | 3 | 1.3 | propranolol (1), metoprolol (1), atenolol (1) |
| C09 | Agents acting on the renin-angiotensin system | 3 | 1.3 | enalapril (3) |
| G04 | Urologicals | 3 | 1.3 | propiverine (2), trospium (1) |
| J01 | Antibacterials for systemic use | 3 | 1.3 | sulfamethoxazole and trimethoprim (1), phenoxymethylpenicillin (1), nitrofurantoin (1) |
| M01 | Anti-inflammatory and anti-rheumatic products | 3 | 1.3 | ibuprofen (3) |
| N07 | Other nervous system drugs | 3 | 1.3 | pyridostigmine (3) |
| V03 | All other therapeutic products | 3 | 1.3 | polystyrene sulfonate (1), calcium folinate (2) |
| Other | 21 | 8.9 | examples: ondansetron (1), metformin (1) | |
| Overall | 237 | 100 |
| Type of Manipulation | n | % |
|---|---|---|
| Tablet split/cut/broken | 80 | 33.8 |
| Tablet split/cut/broken + other manipulation(s) | ||
| Tablet split/cut/broken, then dissolved/suspended/dispersed/diluted in liquid | 35 | 14.8 |
| Tablet split/cut/broken, then dissolved/suspended/dispersed/diluted in liquid, then a proportion of the liquid given | 7 | 3.0 |
| Solid dosage form dissolved/suspended/dispersed | 59 | 24.9 |
| Solid dosage form dissolved/suspended/dispersed + other manipulation(s) | ||
| Tablet dissolved/suspended/dispersed/diluted in liquid, then a proportion of the liquid given | 25 | 10.5 |
| Capsule opened, then content dissolved/suspended/dispersed, then a proportion of the liquid given | 3 | 1.3 |
| Powder for intravenous infusion dissolved in liquid, then a proportion of the liquid given | 3 | 1.3 |
| Other | ||
| Tablet crushed/mortared | 2 | 0.8 |
| Tablet crushed/mortared + other manipulation(s) | 2 | 0.8 |
| Liquid formulation diluted in larger volume | 6 | 2.5 |
| Withdrew a defined volume/dose from a container (ampoule) of liquids for intravenous use | 3 | 1.3 |
| Withdrew a defined volume/dose of powder for oral suspension from a sachet | 2 | 0.8 |
| Counted minitablets | 2 | 0.8 |
| Counted minitablets, then suspended the defined number of minitablets in liquid | 1 | 0.4 |
| Other | 7 | 3.0 |
| Overall | 237 | 100.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahn, J.; Hoerning, A.; Trollmann, R.; Rascher, W.; Neubert, A. Manipulation of Medicinal Products for Oral Administration to Paediatric Patients at a German University Hospital: An Observational Study. Pharmaceutics 2020, 12, 583. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12060583
Zahn J, Hoerning A, Trollmann R, Rascher W, Neubert A. Manipulation of Medicinal Products for Oral Administration to Paediatric Patients at a German University Hospital: An Observational Study. Pharmaceutics. 2020; 12(6):583. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12060583
Chicago/Turabian StyleZahn, Julia, André Hoerning, Regina Trollmann, Wolfgang Rascher, and Antje Neubert. 2020. "Manipulation of Medicinal Products for Oral Administration to Paediatric Patients at a German University Hospital: An Observational Study" Pharmaceutics 12, no. 6: 583. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12060583