Nanotechnology in Modern Photodynamic Therapy of Cancer: A Review of Cellular Resistance Patterns Affecting the Therapeutic Response
Abstract
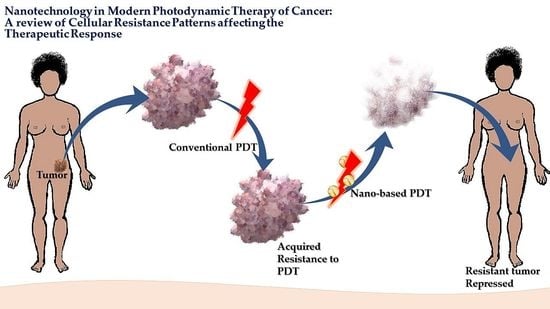
:1. Introduction
2. Photodynamic Therapy
2.1. Basic Pharmacokinetic and Pharmacodynamics of PDT
2.2. Mechanisms of Resistance in PDT
3. Nanotechnology in PDT
3.1. Attenuating Cellular Resistance Using Nanotechnology
3.1.1. Modulation of the PS Uptake and/or Subcellular Localization
3.1.2. Enhanced Damage Repair and Evasion of Apoptosis
3.1.3. Enhanced Drug Efflux
3.1.4. Resistance from Other Factors Other Than Cellular Mechanisms
- Catalase-like activities to decompose H2O2 to O2
- Glutathione consumption for enhancing PDT efficacy
- Increased PS dose
- AS1411 aptamer for nuclear targeting
- Excellent stability
4. Pharmacokinetic Pitfalls in Nanomedicine
4.1. Nanoparticles and Formation of Protein Corona
4.2. Nanoparticles and the Immune System
4.3. Nanoparticles and Their Toxicity to Healthy Tissue
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramaswami, R.; Harding, V.; Newsom-Davis, T. Novel cancer therapies: Treatments driven by tumour biology. Postgrad. Med. J. 2013, 89, 652–658. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wu, L.; Tong, R. PD-1/PD-L1 Inhibitors in Cervical Cancer. Front. Pharmacol. 2019, 10, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordeiro, M.N.; De Lima, R.C.P.; Paolini, F.; da Silva Melo, A.R.; Campos, A.P.F.; Venuti, A.; De Freitas, A.C. Current research into novel therapeutic vaccines against cervical cancer. Expert Rev. Anticancer Ther. 2018, 18, 365–376. [Google Scholar] [CrossRef]
- Giancotti, F.G. Deregulation of Cell Signaling in Cancer. FEBS Lett. 2014, 588, 2558–2570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibata, C.H.; Colussi, V.C.; Oleinick, N.L.; Kinsella, T.J. Photodynamic therapy: A new concept in medical treatment. Braz. J. Med. Biol. Res. 2000, 33, 869–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shishkova, N.; Kuznetsova, O.; Berezov, T. Photodynamic therapy for gynecological diseases and breast cancer. Cancer Biol. Med. 2012, 9, 9–17. [Google Scholar]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell death pathways in photodynamic therapy of cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef] [Green Version]
- Baskaran, R.; Lee, J.; Yang, S.G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef]
- Calixto, G.M.; Bernegossi, J.; de Freitas, L.M.; Fontana, C.R.; Chorilli, M. Nanotechnology Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef]
- Chizenga, E.P.; Chandran, R.; Abrahamse, H. Photodynamic therapy of cervical cancer by eradication of cervical cancer cells and cervical cancer stem cells. Oncotarget 2019, 10, 4380–4396. [Google Scholar] [CrossRef] [PubMed]
- Edrei, R.; Gottfried, V.; Van Lier, J.E.; Kimel, S. Sulfonated phthalocyanines: Photophysical properties, in vitro cell uptake and structure-activity relationships. J. Porphyr. Phthalocyanines 1998, 2, 191–199. [Google Scholar] [CrossRef]
- Nyokong, T. Effects of substituents on the photochemical and photophysical properties of main group metal phthalocyanines. Coord. Chem. Rev. 2007, 251, 1707–1722. [Google Scholar] [CrossRef]
- Rodríguez-Arco, L.; López-López, M.T.; González-Caballero, F.; Durán, J.D.G. Steric repulsion as a way to achieve the required stability for the preparation of ionic liquid-based ferrofluids. J. Colloid Interface Sci. 2011, 357, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.; Di Venosa, G.; Hasan, T.; Batlle, A.I. Mechanisms of resistance to photodynamic therapy. Curr. Med. Chem. 2011, 18, 2486–2515. [Google Scholar] [CrossRef] [Green Version]
- Misra, R.; Acharya, S.; Sahoo, S.K. Cancer nanotechnology: Application of nanotechnology in cancer therapy. Drug. Discov. Today 2010, 15, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Brancaleon, L.; Moseley, H. Laser and Non-laser Light Sources for Photodynamic Therapy. Lasers Med. Sci. 2002, 17, 173–186. [Google Scholar] [CrossRef]
- Henderson, B.W.; Dougherty, T.J. How does photodynamic therapy work? Photochem. Photobiol. 1992, 55, 145–157. [Google Scholar] [CrossRef]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.-Y.; Dai, T.; Hamblin, M.R. Photodynamic therapy for infections: Clinical applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef] [Green Version]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Ding, H.; Yu, H.; Dong, Y.; Tian, R.; Huang, G.; Boothman, D.A.; Sumer, B.D.; Gao, J. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J. Control. Release 2011, 156, 276–280. [Google Scholar] [CrossRef] [Green Version]
- Mroz, P.; Hashmi, J.T.; Huang, Y.Y.; Lange, N.; Hamblin, M.R. Stimulation of anti-tumor immunity by photodynamic therapy. Expert. Rev. Clin. Immunol. 2011, 7, 75–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part three-Photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagnosis Photodyn. Ther. 2005, 2, 91–106. [Google Scholar] [CrossRef] [Green Version]
- Allison, R.R.; Moghissi, K. Photodynamic therapy mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef]
- Xue, L.Y.; Qiu, Y.; He, J.; Kung, H.J.; Oleinick, N.L. Etk/Bmx, a PH-domain-containing tyrosine kinase, protects prostate cancer cells from apoptosis induced by photodynamic therapy or thapsigargin. Oncogene 1999, 18, 3391–3398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, S.; Kochevar, I.E. Singlet oxygen-induced activation of Akt/protein kinase B is independent of growth factor receptors. Photochem. Photobiol. 2013, 78, 361–371. [Google Scholar]
- Espada, J.; Galaz, S.; Sanz-Rodríguez, F.; Blázquez-Castro, A.; Stockert, J.C.; Bagazgoitia, L.; Jaén, P.; González, S.; Cano, A.; Juarranz, A. Oncogenic H-Ras and PI3K signaling can inhibit E-cadherin dependent apoptosis and promote cell survival after photodynamic therapy in mouse keratinocytes. J. Cell Physiol. 2013, 219, 4–93. [Google Scholar] [CrossRef]
- Kocanova, S.; Buytaert, E.; Matroule, J.Y.; Piette, J.; Golab, J.; de Witte, P.; Agostinis, P. Induction of heme-oxygenase 1 requires the p38MAPK and PI3K pathways and suppresses apoptotic cell death following hypericin-mediated photodynamic therapy. Apoptosis 2007, 12, 731–741. [Google Scholar] [CrossRef]
- Tong, Z.; Singh, G.; Rainbow, A.J. Sustained activation of the extracellular signal-regulated kinase pathway protects cells from photofrin-mediated photodynamic therapy. Cancer Res. 2002, 62, 5528–5535. [Google Scholar]
- Srivastava, M.; Ahmad, H.; Gupta, S.; Mukhtar, H. Involvement of Bcl-2 and Bax in photodynamic therapy mediated apoptosis. J. Biol. Chem. 2001, 276, 15481–15488. [Google Scholar] [CrossRef] [Green Version]
- Davids, L.M.; Kleemann, B.; Cooper, S.; Kidson, S.H. Melanomas display increased cytoprotection to hypericin-mediated cytotoxicity through the induction of autophagy. Cell Biol. Int. 2009, 33, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Dewaele, M.; Martinet, W.; Rubio, N.; Verfaillie, T.; de Witte, P.A.; Piette, J.; Agostinis, P. Autophagy pathways activated in response to PDT contribute to cell resistance against ROS damage. J. Cell. Mol. Med. 2011, 15, 1402–1414. [Google Scholar] [PubMed] [Green Version]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one-photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef] [Green Version]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part two-cellular signaling, cell metabolism and modes of cell death. Photodiagn. Photodyn. 2005, 2, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Zhang, A.J.; Chen, M.; Liu, J.J. ABC transporter inhibitors in reversing multidrug resistance to chemotherapy. Curr. Drug Targets 2015, 16, 1356–1371. [Google Scholar] [CrossRef] [PubMed]
- Alison, M.R.; Lin, W.R.; Lim, S.M.; Nicholson, L.J. Cancer stem cells: In the line of fire. Cancer Treat. Rev. 2012, 38, 589–598. [Google Scholar] [CrossRef]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Rycaj, K.; Tang, D.G. Cancer stem cells and radioresistance. Int. J. Radiat. Biol. 2014, 90, 615–621. [Google Scholar] [CrossRef] [Green Version]
- Doherty, M.R.; Smigiel, J.M.; Junk, D.J.; Jackson, M.W. Cancer stem cell plasticity drives therapeutic resistance. Cancers 2016, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Eramo, A.; Ricci-Vitiani, L.; Pallini, A.R.; Lotti, F.; Sette, G.; Pilozzi, E.; Larocca, L.M.; Peschle, C.; De Maria, R. Chemotherapy resistance of glioblastoma stem cells. Cell Death Differ. 2006, 13, 1238–1241. [Google Scholar] [CrossRef] [Green Version]
- Kessel, D.; Woodburn, K.; Skalkos, D. Impaired accumulation of a cationic photosensitizing agent by a cell line exhibiting multidrug resistance. Photochem. Photobiol. 1994, 60, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, Y.; Usuda, J.; Imai, K.; Kubota, M.; Maehara, S.; Ohtani, K. The expression of BCRP/ABCG2 causes resistance to Photofrin-PDT. Jpn. J. Laser Surg. Med. 2008, 28, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Martin, P.M.; Miyauchi, S.; Ananth, S.; Herdman, A.V.; Martindale, R.G.; Podolsky, R.; Ganapathy, V. Down-regulation of BCRP/ABCG2 in colorectal and cervical cancer. Biochem. Biophys. Res. Commun. 2006, 343, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Steadman, K.; Polgar, O.; Morisaki, K.; Blayney, M.; Mistry, P.; Bates, S.E. Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res. 2004, 64, 1242–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selbo, P.K.; Weyergang, A.; Bonsted, A.; Bown, S.G.; Berg, K. Photochemical internalization of therapeutic macromolecular agents: A novel strategy to kill multidrug-resistant cancer cells. J. Pharmacol. Exp. Ther. 2006, 319, 604–612. [Google Scholar] [CrossRef]
- Gomer, C.; Ryter, S.; Ferrario, A.; Rucker, N.; Wong, S.; Fisher, A. Photodynamic Therapy-mediated oxidative stress can induce the expression of heat shock proteins. Cancer Res. 1996, 56, 2355–2360. [Google Scholar]
- Singh, K.K.; Russell, J.; Sigala, B.; Zhang, Y.; Williams, J.; Keshav, K.F. Mitochondrial DNA determines the cellular response to cancer therapeutic agents. Oncogene 1999, 18, 6641–6646. [Google Scholar] [CrossRef] [Green Version]
- Luna, M.; Gomer, C. Isolation and initial characterization of mouse tumor cells resistant to porphyrinmediated photodynamic therapy. Cancer Res. 1991, 51, 4243–4249. [Google Scholar]
- Casas, A.; Perotti, C.; Ortel, B.; Di Venosa, G.; Saccoliti, M.; Batlle, A.; Hasan, T. Tumor cell lines resistant to ALA-mediated photodynamic therapy and possible tools to target surviving cells. Int. J. Oncol. 2006, 29, 397–405. [Google Scholar] [CrossRef] [Green Version]
- Madsen, S.J.; Sun, C.H.; Tromberg, B.J.; Hirschberg, H. Repetitive 5-aminolevulinic acid-mediated photodynamic therapy on human glioma spheroids. J. Neurooncol. 2003, 62, 243–250. [Google Scholar] [CrossRef]
- Kessel, D.; Luo, Y.; Deng, Y.; Chang, C.K. The role of subcellular localization in initiation of apoptosis by photodynamic therapy. Photochem. Photobiol. 1997, 65, 422–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharkey, S.; Wilson, B.; Moorehead, R.; Singh, G. Mitochondrial alterations in Photodynamic Therapy resistant cells. Cancer Res. 1993, 53, 4994–4999. [Google Scholar] [PubMed]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, H.H.; Huang, C.Y.; Chou, C.W.; Makondi, P.T.; Huang, M.T.; Wei, P.L.; Chang, Y.J. Heat shock protein 27 influences the anti-cancer effect of curcumin in colon cancer cells through ROS production and autophagy activation. Life Sci. 2018, 209, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Samali, A.; Cotter, T.G. Heat shock proteins increase resistance to apoptosis. Exp. Cell Res. 1996, 223, 163–170. [Google Scholar] [CrossRef]
- Ikwegbue, P.C.; Masamba, P.; Oyinloye, B.E.; Kappo, A.P. Roles of Heat Shock Proteins in Apoptosis, Oxidative Stress, Human Inflammatory Diseases, and Cancer. Pharmaceuticals 2018, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Nonaka, M.; Ikeda, H.; Inokuchi, T. Inhibitory Effect of Heat Shock Protein 70 on Apoptosis Induced by Photodynamic Therapy in vitro. Photochem. Photobiol. 2004, 79, 94–98. [Google Scholar] [CrossRef]
- Benz, C.C.; Yau, C. Ageing, oxidative stress and cancer: Paradigms in parallax. Nat. Rev. Cancer 2008, 8, 875–879. [Google Scholar] [CrossRef]
- Ji, Z.; Yang, G.; Shahzidi, S.; Tkacz-Stachowska, K.; Suo, Z.; Nesland, J.M.; Peng, Q. Induction of hypoxia-inducible factor-1α overexpression by cobalt chloride enhances cellular resistance to photodynamic therapy. Cancer Lett. 2006, 244, 182–189. [Google Scholar] [CrossRef]
- Mayhew, S.; Vernon, D.; Schofield, J.; Griffiths, J.; Brown, S. Investigation of cross-resistance to a range of photosensitizers, hyperthermia and UV light in two radiation-induced fibrosarcoma cell strains resistant to photodynamic therapy in vitro. Photochem. Photobiol. 2001, 73, 39–46. [Google Scholar] [CrossRef]
- Bernegossi, J.; Calixto, G.; Santos, B.F.; Aida, K.L.; Negrini, T.C.; Duque, C.; Gremião, M.P.D.; Chorilli, M. Highlights in peptide nanoparticle carriers intended to oral diseases. Curr. Top. Med. Chem. 2015, 15, 345–355. [Google Scholar]
- Gao, X.; Yang, L.; Petros, J.A.; Marshall, F.F.; Simons, J.W.; Nie, S. In vivo molecular and cellular imaging with quantum dots. Curr. Opin. Biotechnol. 2005, 16, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Morosini, V.; Bastogne, T.; Frochot, C.; Schneider, R.; François, A.; Guillemin, F.; Barberi-Heyob, M. Quantum dot–folic acid conjugates as potential photosensitizers in photodynamic therapy of cancer. Photochem. Photobiol. Sci. 2011, 10, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Ruoff, R.S.; Qian, D.; Kam Liu, W. Mechanical properties of carbon nanotubes: Theoretical predictions and experimental measurements. C. R. Phys. 2003, 4, 993–1008. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.; Wu, F.; Yuan, P.; Chi, C.; Zhou, N. Magnetic and fluorescent carbon nanotubes for dual modal imaging and photothermal and chemo-therapy of cancer cells in living mice. Carbon 2017, 123, 70–83. [Google Scholar] [CrossRef]
- Caminade, A.M.; Turrin, C.O.; Majoral, J.P. Biological properties of water-soluble phosphorhydrazone dendrimers. Braz. J. Pharm. Sci. 2011, 49, 33–44. [Google Scholar] [CrossRef]
- Narsireddy, A.; Vijayashree, K.; Adimoolam, M.G.; Manorama, S.V.; Rao, N.M. Photosensitizer and peptide-conjugated pamam dendrimer for targeted in vivo photodynamic therapy. Int. J. Nanomed. 2015, 10, 6865–6878. [Google Scholar]
- Kobayashi, H.; Brechbiel, M.W. Dendrimer-based macromolecular MRI contrast agents: Characteristics and applications. Mol. Imaging 2003, 2, 1–10. [Google Scholar] [CrossRef]
- Derycke, A.S.; Kamuhabwa, A.; Gijsens, A.; Roskams, T.; de Vos, D.; Kasran, A.; Huwyler, J.; Missiaen, L.; de Witte, P.A. Transferrin-conjugated liposome targeting of photosensitizer alpcs4 to rat bladder carcinoma cells. J. Natl. Cancer Inst. 2004, 96, 1620–1630. [Google Scholar] [CrossRef] [Green Version]
- Torchillin, V. Antibody-modified liposomes for cancer chemotherapy. Expert Opin. Drug Deliv. 2008, 5, 1003–1025. [Google Scholar] [CrossRef]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef]
- Lopes, L.B.; Speretta, F.F.F.; Bentley, M.V.L.B. Enhancement of skin penetration of vitamin K using monoolein-based liquid crystalline systems. Eur. J. Pharm. Sci. 2007, 32, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Calixto, G.; Bernegossi, J.; Fonseca-Santos, B.; Chorilli, M. Nanotechnology-based drug delivery systems for treatment of oral cancer: A review. Int. J. Nanomed. 2014, 9, 3719–3735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bovis, M.J.; Woodhams, J.H.; Loizidou, M.; Scheglmann, D.; Bown, S.G.; MacRobert, A.J. Improved in vivo delivery of m-thpc via pegylated liposomes for use in photodynamic therapy. J. Control. Release 2012, 157, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Luna, M.; Ferrario, A.; Rucker, N.; Gomer, C. Decreased expression and function of alpha-2 macroglobulin receptor/low density lipoprotein receptor-related protein in photodynamic therapy resistant mouse tumor cells. Cancer Res. 1995, 55, 1820–1823. [Google Scholar]
- Park, E.; Shim, H.; Lee, G.; Kim, J.H.; Kim, D.W. Comparison of toxicity between the different-type TiO2 nanowires in vivo and in vitro. Arch. Toxicol. 2013, 87, 1219–1230. [Google Scholar] [CrossRef]
- Naidoo, C.; Kruger, C.A.; Abrahamse, H. Targeted photodynamic therapy treatment of in vitro A375 metastatic melanoma cells. Oncotarget 2019, 10, 6079–6095. [Google Scholar] [CrossRef] [Green Version]
- Muehlmann, L.A.; Rodrigues, M.C.; Figueiró Longo, J.P.; Garcia, M.P.; Py-Daniel, K.R.; Veloso, A.B.; de Souza, P.E.N.; da Silva, S.W.; Azevedo, R.B. Aluminium-phthalocyanine chloride nanoemulsions for anticancer photodynamic therapy: Development and in vitro activity against monolayers and spheroids of human mammary adenocarcinoma MCF-7 cells. J. Nanobiotechnol. 2015, 13, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Nombona, N.; Antunes, E.; Chidawanyika, W.; Kleyi, P.; Tshentu, Z.; Nyokong, T. Synthesis, photophysics and photochemistry of phthalocyanine-ɛ-polylysine conjugates in the presence of metal nanoparticles against Staphylococcus aureus. J. Photochem. Photobiol. A Chem. 2012, 233, 24–33. [Google Scholar] [CrossRef]
- Stuchinskaya, T.; Moreno, M.; Cook, M.J.; Edwards, D.R.; Russell, D.A. Targeted photodynamic therapy of breast cancer cells using antibody-phthalocyanine-gold nanoparticle conjugates. Photoch. Photobiol. Sci. 2011, 10, 822–831. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaki, S.; Serada, S.; Hiramatsu, K.; Nojima, S.; Matsuzaki, S.; Ueda, Y.; Ohkawara, T.; Mabuchi, S.; Fujimoto, M.; Morii, E.; et al. Anti-glypican-1 antibody-drug conjugate exhibits potent preclinical antitumor activity against glypican-1 positive uterine cervical cancer. Int. J. Cancer 2018, 142, 1056–1066. [Google Scholar] [CrossRef]
- Lakhin, A.V.; Tarantul, V.Z.; Gening, L.V. Aptamers: Problems, solutions and prospects. Acta Naturae 2013, 5, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Nimjee, S.M.; Rusconi, C.P.; Sullenger, B.A. Aptamers: An emerging class of therapeutics. Annu. Rev. Med. 2005, 56, 555–583. [Google Scholar] [CrossRef]
- Mallikaratchy, P.; Tang, Z.; Tan, W. Cell specific aptamer-photosensitizer conjugates as a molecular tool in photodynamic therapy. Chem. Med. Chem. 2008, 3, 425–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Park, W.; Kim, D.; Lee, E.S.; Lee, D.H.; Jeong, S.; Park, J.M.; Na, K. Tumor-Specific Aptamer-Conjugated Polymeric Photosensitizer for Effective Endo-Laparoscopic Photodynamic Therapy. Adv. Funct. Mater. 2019, 29, 1900084. [Google Scholar] [CrossRef]
- Shieh, Y.-A.; Yang, S.-J.; Wei, M.-F.; Shieh, M.-J. Aptamer-Based Tumor-Targeted Drug Delivery for Photodynamic Therapy. ACS Nano 2010, 4, 1433–1442. [Google Scholar] [CrossRef]
- Paunovic, J.; Vucevic, D.; Radosavljevic, T.; Mandić-Rajčević, S.; Pantic, I. Iron-based nanoparticles and their potential toxicity: Focus on oxidative stress and apoptosis. Chem. Biol. Interact. 2020, 316, 108935. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Choi, D.-H.; Kim, Y.; Lee, E.-W.; Song, J.; Cho, M.-H.; Kim, J.-H.; Kim, S.-W. Magnetic iron oxide nanoparticles induce autophagy preceding apoptosis through mitochondrial damage and ER stress in RAW264.7 cells. Toxicol. In Vitro 2014, 28, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Marí, M.; Morales, A.; Colell, A.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal. 2009, 11, 2685–2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Qian, S.; Schafer, F.; Domann, F.; Oberley, L.; Buettner, G. Phospholipid hydroperoxide glutathione peroxidase protects against singlet oxygen-induced cell damage of photodynamic therapy. Free Radic. Biol. Med. 2001, 30, 825–835. [Google Scholar] [CrossRef]
- Dolgachev, V.; Oberley, L.W.; Huang, T.T.; Kraniak, J.M.; Tainsky, M.A.; Hanada, K.; Separovic, D.A. Role for manganese superoxide dismutase in apoptosis after photosensitization. Biochem. Biophys. Res. Commun. 2005, 332, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Oberdanner, C.B.; Plaetzer, K.; Kiesslich, T.; Krammer, B. Photodynamic treatment with fractionated light decreases production of reactive oxygen species and cytotoxicity in vitro via regeneration of glutathione. Photochem. Photobiol. 2005, 81, 609–613. [Google Scholar] [CrossRef]
- Theodossiou, T.A.; Olsen, C.E.; Jonsson, M.; Kubin, A.; Hothersall, J.S.; Berg, K. The diverse roles of glutathione-associated cell resistance against hypericin photodynamic therapy. Redox Biol. 2017, 12, 191–197. [Google Scholar] [CrossRef]
- Ling, X.; Chen, X.; Riddell, I.A.; Tao, W.; Wang, J.; Hollett, G.; Lippard, S.J.; Farokhzad, O.C.; Shi, J.; Wu, J. Glutathione-Scavenging Poly(disulfide amide) Nanoparticles for the Effective Delivery of Pt(IV) Prodrugs and Reversal of Cisplatin Resistance. Nano Lett. 2018, 18, 4618–4625. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.; Iwakoshi, N.N.; Anderson, K.C.; Glimcher, L.H. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc. Natl. Acad. Sci. USA 2003, 100, 9946–9951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szokalska, A.; Makowski, M.; Nowis, D.; Wilczynski, G.M.; Kujawa, M.; Wójcik, C.; Mlynarczuk-Bialy, I.; Salwa, P.; Bil, J.; Janowska, S.; et al. Proteasome inhibition potentiates antitumor effects of photodynamic therapy in mice through induction of endoplasmic reticulum stress and unfolded protein response. Cancer Res. 2009, 69, 4235–4243. [Google Scholar] [CrossRef] [Green Version]
- Broekgaarden, M.; Weijer, R.; Krekorian, M.; van den IJssel, B.; Kos, M.; Alles, L.K.; van Wijk, A.C.; Bikadi, Z.; Hazai, E.; van Gulik, T.M.; et al. Inhibition of hypoxia-inducible factor 1 with acriflavine sensitizes hypoxic tumor cells to photodynamic therapy with zinc phthalocyanine-encapsulating cationic liposomes. Nano. Res. 2016, 9, 1639–1662. [Google Scholar] [CrossRef]
- Li, W.; Zhang, W.; Ohnishi, K.; Yamada, I.; Ohno, R.; Hashimoto, K. 5-aminolevulinic acid-mediated photodynamic therapy in multidrug resistant leukemia cells. J. Photochem. Photobiol. 2001, 60, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Baer, M.R.; Bowman, M.J.; Pera, P.; Zheng, X.; Morgan, J.; Pandey, R.A.; Oseroff, A.R. The tyrosine kinase inhibitor imatinib mesylate enhances the efficacy of photodynamic therapy by inhibiting ABCG2. Clin. Cancer Res. 2007, 13, 2463–2470. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Dong, Z.; Fu, T.; Liu, J.; Chen, Q.; Li, Y.; Zhu, R.; Xu, L.; Liu, Z. Modulation of Hypoxia in Solid Tumor Microenvironment with MnO2 Nanoparticles to Enhance Photodynamic Therapy. Adv. Funct. Mater. 2016, 26, 5490–5498. [Google Scholar] [CrossRef]
- Lin, T.; Zhao, X.; Zhao, S.; Yu, H.; Cao, W.; Chen, W.; Wei, H.; Guo, H. O2-generating MnO2 nanoparticles for enhanced photodynamic therapy of bladder cancer by ameliorating hypoxia. Theranostics 2018, 8, 990–1004. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Yan, S.; Chen, P.; Du, W.; Liu, B.F. Modulation of tumor microenvironment by metal-organic-framework-derived nanoenzyme for enhancing nucleus-targeted photodynamic therapy. Nano Res. 2020, in press. [Google Scholar] [CrossRef]
- Anderson, N.L.; Anderson, N.G. The Human Plasma Proteome. Mol. Cell. Proteomics. 2002, 1, 845–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, V.H.; Lee, B.J. Protein corona: A new approach for nanomedicine design. Int. J. Nanomed. 2017, 12, 3137–3151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding Biophysicochemical Interactions at the Nano-Bio Interface. Nat. Mater. 2009, 8, 543–555. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, B. Self-Assembled Nanoparticles Based on PEGylated Conjugated Polyelectrolyte and Drug Molecules for Image-Guided Drug Delivery and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2014, 6, 14903–14910. [Google Scholar] [CrossRef]
- Mirshafiee, V.; Kim, R.; Park, S.; Mahmoudi, M.; Kraft, M.L. Impact of protein pre-coating on the protein corona composition and nanoparticle cellular uptake. Biomaterials 2016, 75, 295–304. [Google Scholar] [CrossRef]
- Saha, K.; Rahimi, M.; Yazdani, M.; Kim, S.T.; Moyano, D.F.; Hou, S.R.; Das, R.; Mout, R.; Rezaee, F.; Mahmoudi, M.; et al. Regulation of Macrophage Recognition through the Interplay of Nanoparticle Surface Functionality and Protein Corona. ACS Nano 2016, 10, 4421–4430. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-F.; Mäkilä, E.M.; Bonduelle, C.; Rytkönen, J.; Raula, J.; Almeida, S.; Närvänen, A.; Salonen, J.J.; Lecommandoux, S.; Hirvonen, J.T.; et al. Functionalization of Alkyne-Terminated Thermally Hydrocarbonized Porous Silicon Nanoparticles With Targeting Peptides and Antifouling Polymers: Effect on the Human Plasma Protein Adsorption. ACS Appl. Mater. Interfaces 2015, 7, 2006–2015. [Google Scholar] [CrossRef]
- Safavi-Sohi, R.; Maghari, S.; Raoufi, M.; Jalali, S.A.; Hajipour, M.J.; Ghassempour, A.; Mahmoudi, M. Bypassing Protein Corona Issue on Active Targeting: Zwitterionic Coatings Dictate Specific Interactions of Targeting Moieties and Cell Receptors. ACS Appl. Mater. Interfaces 2016, 8, 22808–22818. [Google Scholar] [CrossRef] [PubMed]
- Chanan-Khan, A.; Szebeni, J.; Savay, S.; Liebes, L.; Rafique, N.M.; Alving, C.R.; Muggia, F.M. Complement activation following first exposure to PEGylated liposomal doxorubicin (Doxil): Possible role in hypersensitivity reactions. Ann. Oncol. 2003, 14, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Schrand, A.M.; Rahman, M.F.; Hussain, S.M.; Schlager, J.J.; Smith, D.A.; Syed, A.F. Metal-based nanoparticles and their toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 5, 544–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.; Seaton, A.; Stone, V.; Brown, S.; Macnee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestoslike pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.H.; Chang, L.W.; Lin, P. Metal-Based Nanoparticles and the Immune System: Activation, Inflammation, and Potential Applications. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Zolnik, B.S.; González-Fernández, A.; Sadrieh, N.; Dobrovolskaia, M.A. Nanoparticles and the immune system. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Mankus, C.I.; Vermilya, A.M.; Soheilian, F.; Clogston, J.D.; Dobrovolskaia, M.A. Feraheme® suppresses immune function of human T lymphocytes through mitochondrial damage and mitoROS production. Toxicol. Appl. Pharmacol. 2018, 350, 52–63. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.; Opatz, T.; Landfester, K.; Wurm, F. Carbohydrate nanocarriers in biomedical applications: Functionalization and construction. Chem. Soc. Rev. 2015, 44, 8301–8325. [Google Scholar] [CrossRef] [Green Version]
- Nuhn, L.; Barz, M.; Zentel, R. New perspectives of HPMA-based copolymers derived by post-polymerization modification (feature). Macromol. Biosci. 2014, 14, 607–618. [Google Scholar] [CrossRef]
- Klinker, K.; Barz, M. Polypeptoides: Hybrid systems based on polypeptides and polypeptoids (feature). Macromol. Rapid Comm. 2015, 36, 1943–1957. [Google Scholar] [CrossRef]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Rahgozar, S.; Zarrabi, A. Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation. BMC Neurosci. 2017, 18, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sripetchwandee, J.; Wongjaikam, S.; Krintratun, W.; Chattipakorn, N.; Chattipakorn, S.C. A combination of an iron chelator with an antioxidant effectively diminishes the dendritic loss, tau-hyperphosphorylation, amyloids-β accumulation and brain mitochondrial dynamic disruption in rats with chronic iron-overload. Neuroscience 2016, 332, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Hare, D.J.; Double, K.L. Iron and dopamine: A toxic couple. Brain 2016, 139, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Youssef, Z.; Vanderesse, R.; Colombeau, L.; Baros, F.; Roques-Carmes, T.; Frochot, C.; Wahab, H.; Toufaily, J.; Hamieh, T.; Acherar, S.; et al. The application of titanium dioxide, zinc oxide, fullerene, and graphene nanoparticles in photodynamic therapy. Cancer Nanotechnol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Nadhman, A.; Nazir, S.; Khan, M.I.; Arooj, S.; Bakhtiar, M.; Shahnaz, G.; Yasinzai, M. PEGylated silver doped zinc oxide nanoparticles as novel photosensitizers for photodynamic therapy against Leishmania. Free Radic. Biol. Med. 2014, 77, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.-N.; Yoon, T.-J.; Minai-Tehrani, A.; Kim, J.-E.; Park, S.J.; Jeong, M.S.; Ha, S.-W.; Lee, J.-K.; Kim, J.S.; Cho, M.-H. Zinc oxide nanoparticle induced autophagic cell death and mitochondrial damage via reactive oxygen species generation. Toxicol. In Vitro 2013, 27, 1187–1195. [Google Scholar] [CrossRef] [PubMed]


| Proposed Mechanism(s) | Cell Line | Photosensitizer(s) | Reference |
|---|---|---|---|
| MDR Mediated drug efflux * | P388/ADR murine leukemia | Copper benzochlorin iminium salt, a cationic PS | [41] |
| ABCG2 associated drug efflux * | NCI-H1650 MX50 bronchoalveolar carcinoma | Pyropheophorbide | [42,43,44] |
| Chlorin e6 | |||
| PpIX from 5-aminolevulinic acid (ALA) | |||
| Endocytic vesicle localization of TPPS2a | MES-SA/Dx5 cells | Disulfonated meso-tetraphenylporphine (TPPS2a) | [45] |
| Modulation of the PS uptake and/or subcellular localization as well as changes in mitochondrial size and function | RIF-1 fibrosarcoma | Photofrin II | [46,47,48] |
| Polyhematoporphyrin (PHP) | |||
| Zinc (II) pyridinium-substituted phthalocyanine (ZnPCP) | |||
| Alterations in the enzymes of the heme pathway that produces PpIX | murine mammary adenocarcinoma | 5-aminolevulinic acid (ALA) | [49] |
| Attenuation of light in tissue LD | human glioma spheroids | 5-aminolevulinic acid (ALA) | [50] |
| Delayed apoptotic response sp | P388 murine leukemia | tin octaethylpurpurin amidine (SnOPA) | [51] |
| Nanomaterial | Description | Application | Reference(s) |
|---|---|---|---|
| Nanoparticles (NPs) | NPs are nanosized colloidal particles with a polymeric matrix that can adsorb or bind a therapeutic compound. NPs can be classified as metallic NPs, polymeric NPs (PNPs) and solid lipid NPs (SLNs), depending on the material of which they are made. | Chemotherapy PDT | [61] |
| Quantum dots | Semiconductor particles with an inert polymer coating. The material used for the core can be chosen depending on the emission wavelength range being targeted. Targeted molecules can be attached to the coating. | Cancer imaging PDT | [62,63] |
| Carbon nanotubes | Cylinder-like assemblies of carbon atoms with cross-sectional dimensions in the nanometer range, and lengths that can extend over a thousand times their diameters. | Biomarker detection chemotherapy | [64,65] |
| Dendrimers | These polymers possess an architecture that gives them an alterable size and shape with several branches around an inner core. | PDT Chemotherapy Cancer imaging | [66,67,68] |
| Liposomes | Uni/multilamellar nanosized carrier molecules made of lipids surrounding a water core, formed from the dispersion of phospholipids in an aqueous medium. | PDT Chemotherapy | [69,70] |
| Nanowires and nanocantilever arrays | Nanocantilever are flexible beams that can be coated with molecules capable of binding to cancer biomarkers. When certain biomolecular interactions occur on one surface of a microcantilever beam, the cantilever bends and can be detected. Nanoscale sensing wires that can be coated with molecules such as antibodies to bind to proteins of interest and transmit their information through electrodes to computers. | Biomarker detection Early detection of precancerous and malignant lesions from biological fluids | [71] |
| Liquid Crystalline Systems | Also called anisotropic phase, they are polymers that lie between the boundaries of solid substances and liquids when in melt state, and, macroscopically, in the melt state, they are fluids. | Transdermal delivery of vitamins | [72] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chizenga, E.P.; Abrahamse, H. Nanotechnology in Modern Photodynamic Therapy of Cancer: A Review of Cellular Resistance Patterns Affecting the Therapeutic Response. Pharmaceutics 2020, 12, 632. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12070632
Chizenga EP, Abrahamse H. Nanotechnology in Modern Photodynamic Therapy of Cancer: A Review of Cellular Resistance Patterns Affecting the Therapeutic Response. Pharmaceutics. 2020; 12(7):632. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12070632
Chicago/Turabian StyleChizenga, Elvin Peter, and Heidi Abrahamse. 2020. "Nanotechnology in Modern Photodynamic Therapy of Cancer: A Review of Cellular Resistance Patterns Affecting the Therapeutic Response" Pharmaceutics 12, no. 7: 632. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12070632