Bypassing the Blood–Brain Barrier: Direct Intracranial Drug Delivery in Epilepsies
Abstract
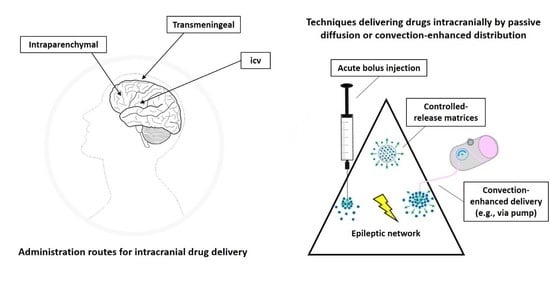
:1. Introduction
2. Technical Considerations
3. Animal Model Aspects
4. Targeting the Seizure Focus
4.1. Intracerebroventricular (Icv) Drug Delivery to Modulate Seizure Focus
4.1.1. Acute Icv Drug Delivery in Animal Models of Seizures/Epilepsies
4.1.2. Chronic Icv Drug Delivery in Animal Models of Seizures/Epilepsies
4.1.3. Icv Drug Delivery in Humans with Drug-Resistant Epilepsy (DRE)
4.2. Transmeningeal Drug Delivery to Modulate Seizure Focus
4.2.1. Acute Transmeningeal Drug Delivery in Animal Models of Seizures/Epilepsies
4.2.2. Chronic Transmeningeal Drug Delivery in Animal Models of Seizures/Epilepsies
4.2.3. Transmeningeal Drug Delivery in Humans with DRE
4.3. Intraparenchymal Drug Delivery to Modulate Seizure Focus
4.3.1. Acute Intraparenchymal Drug Delivery in Animal Models of Seizures/Epilepsies
4.3.2. Chronic Intraparenchymal Drug Delivery in Animal Models of Seizures/Epilepsies
4.3.3. Intraparenchymal Drug Delivery in Humans with DRE
5. Targeting Remote Brain Structures of the Epileptic Network
5.1. Basal Ganglia
5.1.1. Basal Ganglia Anatomical and Pathophysiological Background
5.1.2. Acute Basal Ganglia Drug Delivery in Animal Models of Seizures/Epilepsies
5.1.3. Chronic Basal Ganglia Drug Delivery in Animal Models of Seizures/Epilepsies
5.1.4. Lack of Clinical Trials of Drug Delivery into Basal Ganglia in Humans with DRE
5.2. Thalamic Regions
5.3. Further Regions
6. Challenges
6.1. Drug Resistance
6.2. Pharmacological Tolerance upon Intracranial Drug Delivery
6.3. Differences in Brain Size and Connectivity between Laboratory Animals and Humans
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Keezer, M.R.; Sisodiya, S.M.; Sander, J.W. Comorbidities of epilepsy: Current concepts and future perspectives. Lancet Neurol. 2016, 15, 106–115. [Google Scholar] [CrossRef]
- Grönheit, W.; Popkirov, S.; Wehner, T.; Schlegel, U.; Wellmer, J. Practical Management of Epileptic Seizures and Status Epilepticus in Adult Palliative Care Patients. Front. Neurol. 2018, 9, 595. [Google Scholar] [CrossRef] [PubMed]
- LaPenna, P.; Tormoehlen, L.M. The Pharmacology and Toxicology of Third-Generation Anticonvulsant Drugs. J. Med. Toxicol. 2017, 13, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Kalilani, L.; Sun, X.; Pelgrims, B.; Noack-Rink, M.; Villanueva, V. The epidemiology of drug-resistant epilepsy: A systematic review and meta-analysis. Epilepsia 2018, 59, 2179–2193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, D.; Löscher, W. Drug resistance in epilepsy: Putative neurobiologic and clinical mechanisms. Epilepsia 2005, 46, 858–877. [Google Scholar] [CrossRef]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; Moshe, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- Janmohamed, M.; Brodie, M.J.; Kwan, P. Pharmacoresistance—Epidemiology, mechanisms, and impact on epilepsy treatment. Neuropharmacology 2020, 168, 107790. [Google Scholar] [CrossRef]
- Kehne, J.H.; Klein, B.D.; Raeissi, S.; Sharma, S. The National Institute of Neurological Disorders and Stroke (NINDS) Epilepsy Therapy Screening Program (ETSP). Neurochem. Res. 2017, 42, 1894–1903. [Google Scholar] [CrossRef] [Green Version]
- Dawit, S.; Crepeau, A.Z. When Drugs Do Not Work: Alternatives to Antiseizure Medications. Curr. Neurol. Neurosci. Rep. 2020, 20, 37. [Google Scholar] [CrossRef]
- Sisterson, N.D.; Kokkinos, V. Neuromodulation of Epilepsy Networks. Neurosurg. Clin. N. Am. 2020, 31, 459–470. [Google Scholar] [CrossRef]
- Rugg-Gunn, F.; Miserocchi, A.; McEvoy, A. Epilepsy surgery. Pr. Neurol. 2020, 20, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Gernert, M.; Heinemann, U. Cell and gene therapies in epilepsy--promising avenues or blind alleys? Trends Neurosci. 2008, 31, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Rogawski, M.A. Convection-enhanced delivery in the treatment of epilepsy. Neurotherapeutics 2009, 6, 344–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesraoua, B.; Deleu, D.; Kullmann, D.M.; Shetty, A.K.; Boon, P.; Perucca, E.; Mikati, M.A.; Asadi-Pooya, A.A. Novel therapies for epilepsy in the pipeline. Epilepsy Behav. 2019, 97, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Schachter, S.C.; Schomer, D.L.; Blume, H.; Ives, J.R.; Joseph, J.; Vonsattel, J.-P.; Dinsomore, J. Porcine fetal GABA-producing neural cell transplants for human partial onset seizures: Safety and feasibility. Epilepsia 1998, 39, 67. [Google Scholar]
- Heiss, J.D.; Argersinger, D.P.; Theodore, W.H.; Butman, J.A.; Sato, S.; Khan, O.I. Convection-Enhanced Delivery of Muscimol in Patients with Drug-Resistant Epilepsy. Neurosurgery 2019, 85, E4–E15. [Google Scholar] [CrossRef] [Green Version]
- Spatazza, J.; Mancia Leon, W.R.; Alvarez-Buylla, A. Transplantation of GABAergic interneurons for cell-based therapy. Prog. Brain Res. 2017, 231, 57–85. [Google Scholar] [CrossRef]
- Barcia, J.A.; Gallego, J.M. Intraventricular and intracerebral delivery of anti-epileptic drugs in the kindling model. Neurotherapeutics 2009, 6, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Stacey, W.C.; Litt, B. Technology insight: Neuroengineering and epilepsy-designing devices for seizure control. Nat. Clin. Pr. Neurol. 2008, 4, 190–201. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H.; Sisodiya, S.M.; Vezzani, A. Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options. Pharm. Rev. 2020, 72, 606–638. [Google Scholar] [CrossRef]
- Tishler, D.M.; Weinberg, K.I.; Hinton, D.R.; Barbaro, N.; Annett, G.M.; Raffel, C. MDR1 gene expression in brain of patients with medically intractable epilepsy. Epilepsia 1995, 36, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Kwan, P. The Concept of Drug-Resistant Epileptogenic Zone. Front Neurol. 2019, 10, 558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sisodiya, S.M.; Lin, W.R.; Harding, B.N.; Squier, M.V.; Thom, M. Drug resistance in epilepsy: Expression of drug resistance proteins in common causes of refractory epilepsy. Brain 2002, 125, 22–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potschka, H.; Volk, H.A.; Löscher, W. Pharmacoresistance and expression of multidrug transporter P-glycoprotein in kindled rats. Neuroreport 2004, 15, 1657–1661. [Google Scholar] [CrossRef]
- Volk, H.A.; Löscher, W. Multidrug resistance in epilepsy: Rats with drug-resistant seizures exhibit enhanced brain expression of P-glycoprotein compared with rats with drug-responsive seizures. Brain 2005, 128, 1358–1368. [Google Scholar] [CrossRef]
- Tang, F.; Hartz, A.M.S.; Bauer, B. Drug-Resistant Epilepsy: Multiple Hypotheses, Few Answers. Front. Neurol. 2017, 8, 301. [Google Scholar] [CrossRef]
- Lazarowski, A.; Czornyj, L.; Lubienieki, F.; Girardi, E.; Vazquez, S.; D’Giano, C. ABC transporters during epilepsy and mechanisms underlying multidrug resistance in refractory epilepsy. Epilepsia 2007, 48 (Suppl. S5), 140–149. [Google Scholar] [CrossRef]
- Kerb, R.; Aynacioglu, A.S.; Brockmoller, J.; Schlagenhaufer, R.; Bauer, S.; Szekeres, T.; Hamwi, A.; Fritzer-Szekeres, M.; Baumgartner, C.; Ongen, H.Z.; et al. The predictive value of MDR1, CYP2C9, and CYP2C19 polymorphisms for phenytoin plasma levels. Pharm. J. 2001, 1, 204–210. [Google Scholar] [CrossRef] [Green Version]
- Walker, M.C. Hippocampal Sclerosis: Causes and Prevention. Semin. Neurol. 2015, 35, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Pitkanen, A.; Sutula, T.P. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002, 1, 173–181. [Google Scholar] [CrossRef]
- Aronica, E.; Mühlebner, A. Neuropathology of epilepsy. Handb. Clin. Neurol. 2017, 145, 193–216. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chaitanya, G.; Asma, B.; Caciagli, L.; Bassett, D.S.; Tracy, J.I.; Sperling, M.R. Disrupted basal ganglia-thalamocortical loops in focal to bilateral tonic-clonic seizures. Brain 2020, 143, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Otarula, K.A.; Schuele, S. Networks in Temporal Lobe Epilepsy. Neurosurg. Clin. N. Am. 2020, 31, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Gale, K. Progression and generalization of seizure discharge: Anatomical and neurochemical substrates. Epilepsia 1988, 29 (Suppl. S2), S15–S34. [Google Scholar] [CrossRef]
- Löscher, W.; Ebert, U. Basic mechanisms of seizure propagation: Targets for rational drug design and rational polypharmacy. Epilepsy Res. Suppl. 1996, 11, 17–43. [Google Scholar]
- Vuong, J.; Devergnas, A. The role of the basal ganglia in the control of seizure. J. Neural. Transm. (Vienna) 2018, 125, 531–545. [Google Scholar] [CrossRef]
- Vytvarova, E.; Marecek, R.; Fousek, J.; Strycek, O.; Rektor, I. Large-scale cortico-subcortical functional networks in focal epilepsies: The role of the basal ganglia. Neuroimage Clin. 2017, 14, 28–36. [Google Scholar] [CrossRef]
- Gale, K. Subcortical structures and pathways involved in convulsive seizure generation. J. Clin. Neurophysiol. 1992, 9, 264–277. [Google Scholar] [CrossRef]
- Gernert, M. Intrasubthalamic cell transplants for epilepsy therapy: Hopes and concerns. Neuroreport 2013, 24, 1062–1066. [Google Scholar] [CrossRef]
- Depaulis, A.; Charpier, S. Pathophysiology of absence epilepsy: Insights from genetic models. Neurosci. Lett 2018, 667, 53–65. [Google Scholar] [CrossRef]
- Deransart, C.; Depaulis, A. The control of seizures by the basal ganglia? A review of experimental data. Epileptic Disord. 2002, 4 (Suppl. S3), S61–S72. [Google Scholar] [PubMed]
- Faingold, C.L.; Blumenfeld, H. Targeting Neuronal Networks with Combined Drug and Stimulation Paradigms Guided by Neuroimaging to Treat Brain Disorders. Neuroscientist 2015, 21, 460–474. [Google Scholar] [CrossRef] [PubMed]
- Van Tienderen, G.S.; Berthel, M.; Yue, Z.; Cook, M.; Liu, X.; Beirne, S.; Wallace, G.G. Advanced fabrication approaches to controlled delivery systems for epilepsy treatment. Expert Opin. Drug Deliv. 2018, 15, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Halliday, A.J.; Moulton, S.E.; Wallace, G.G.; Cook, M.J. Novel methods of antiepileptic drug delivery—Polymer-based implants. Adv. Drug Deliv. Rev. 2012, 64, 953–964. [Google Scholar] [CrossRef]
- Fisher, R.S.; Chen, D.K. New routes for delivery of anti-epileptic medications. Acta Neurol. Taiwan 2006, 15, 225–231. [Google Scholar]
- Pathan, S.A.; Jain, G.K.; Akhter, S.; Vohora, D.; Ahmad, F.J.; Khar, R.K. Insights into the novel three ‘D’s of epilepsy treatment: Drugs, delivery systems and devices. Drug Discov. Today 2010, 15, 717–732. [Google Scholar] [CrossRef]
- Ludvig, N. Subdural Pharmacotherapy for the Treatment of Intractable Focal Neocortical Epilepsy. Drug Deliv. Lett. 2012, 2, 70–81. [Google Scholar] [CrossRef]
- Ludvig, N.; Medveczky, G.; French, J.A.; Carlson, C.; Devinsky, O.; Kuzniecky, R.I. Evolution and prospects for intracranial pharmacotherapy for refractory epilepsies: The subdural hybrid neuroprosthesis. Epilepsy Res. Treat. 2010, 2010, 725696. [Google Scholar] [CrossRef]
- Hersh, D.S.; Wadajkar, A.S.; Roberts, N.; Perez, J.G.; Connolly, N.P.; Frenkel, V.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Evolving Drug Delivery Strategies to Overcome the Blood Brain Barrier. Curr. Pharm. Des. 2016, 22, 1177–1193. [Google Scholar] [CrossRef] [Green Version]
- Vogel, T.W.; Bruce, J.N. Local Delivery Methods into the CNS. In Minimally Invasive Neurosurgery; Proctor, M.R., Black, P.M., Eds.; Humana Press: Totowa, NJ, USA, 2005; pp. 297–316. [Google Scholar]
- Lonser, R.R.; Sarntinoranont, M.; Morrison, P.F.; Oldfield, E.H. Convection-enhanced delivery to the central nervous system. J. Neurosurg. 2015, 122, 697–706. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Gu, Q.; Yue, Z.; Crook, J.M.; Moulton, S.E.; Cook, M.J.; Wallace, G.G. Development of drug-loaded polymer microcapsules for treatment of epilepsy. Biomater. Sci. 2017, 5, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Bauquier, S.H.; McLean, K.J.; Jiang, J.L.; Boston, R.C.; Lai, A.; Yue, Z.; Moulton, S.E.; Halliday, A.J.; Wallace, G.; Cook, M.J. Evaluation of the Biocompatibility of Polypyrrole Implanted Subdurally in GAERS. Macromol. Biosci. 2017, 17, 1600334. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci. Lett. 1991, 127, 160–164. [Google Scholar] [CrossRef]
- Madhavan, D.; Mirowski, P.; Ludvig, N.; Carlson, C.; Doyle, W.; Devinsky, O.; Kuzniecky, R. Effects of subdural application of lidocaine in patients with focal epilepsy. Epilepsy Res. 2008, 78, 235–239. [Google Scholar] [CrossRef]
- Gey, L.; Gernert, M.; Löscher, W. Continuous bilateral infusion of vigabatrin into the subthalamic nucleus: Effects on seizure threshold and GABA metabolism in two rat models. Neurobiol. Dis. 2016, 91, 194–208. [Google Scholar] [CrossRef]
- Feja, M.; Deking, L.S.; Gernert, M. Chronic convection-enhanced muscimol delivery into the subthalamic nucleus produces transient anticonvulsant effects in an acute rat seizure model. ScienceOpen Posters 2020. [Google Scholar] [CrossRef]
- Serralta, A.; Barcia, J.A.; Ortiz, P.; Duran, C.; Hernandez, M.E.; Alos, M. Effect of intracerebroventricular continuous infusion of valproic acid versus single i.p. and i.c.v. injections in the amygdala kindling epilepsy model. Epilepsy Res. 2006, 70, 15–26. [Google Scholar] [CrossRef]
- Barcia, J.A.; Castillo, A.; Gutierrez, R.; Gallego, J.M.; Ortiz, L.; Hernandez, M.E. Continuous intra-amygdalar infusion of GABA in the amygdala kindling model of epilepsy in rat. Epilepsy Res. 2004, 58, 19–26. [Google Scholar] [CrossRef]
- Van Dycke, A.; Raedt, R.; Dauwe, I.; Sante, T.; Wyckhuys, T.; Meurs, A.; Vonck, K.; Wadman, W.; Boon, P. Continuous local intrahippocampal delivery of adenosine reduces seizure frequency in rats with spontaneous seizures. Epilepsia 2010, 51, 1721–1728. [Google Scholar] [CrossRef]
- Gallego, J.M.; Ortiz, L.; Gutierrez, R.; Barcia, J.A. Continuous bilateral infusion of GABA in the dorsomedian nucleus of the thalamus elevates the generalized seizure threshold in amygdala-kindled rats. Seizure 2009, 18, 537–540. [Google Scholar] [CrossRef] [Green Version]
- Cook, M.; Murphy, M.; Bulluss, K.; D’Souza, W.; Plummer, C.; Priest, E.; Williams, C.; Sharan, A.; Fisher, R.; Pincus, S.; et al. Anti-seizure therapy with a long-term, implanted intra-cerebroventricular delivery system for drug-resistant epilepsy: A first-in-man study. EClinicalMedicine 2020, 22, 100326. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.; Lonser, R.R.; Morrison, P.F.; Governale, L.S.; Oldfield, E.H. Variables affecting convection-enhanced delivery to the striatum: A systematic examination of rate of infusion, cannula size, infusate concentration, and tissue-cannula sealing time. J. Neurosurg. 1999, 90, 315–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, V.M.; Rechberger, J.S.; Himes, B.T.; Daniels, D.J. The 100 Most-Cited Articles About Convection-Enhanced Delivery to the Brain: A Bibliometric Analysis. World Neurosurg. 2019, 129, 497–502.e496. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.M.; French, J.A.; Kuzniecky, R.I.; Devinsky, O.; Ludvig, N. Periodic transmeningeal muscimol maintains its antiepileptic efficacy over three weeks without inducing tolerance, in rats. Neurosci. Lett. 2011, 494, 135–138. [Google Scholar] [CrossRef]
- Dümpelmann, M. Early seizure detection for closed loop direct neurostimulation devices in epilepsy. J. Neural. Eng. 2019, 16, 041001. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.G.; Eder, H.G.; Blum, D.E.; Drachev, A.; Fisher, R.S. An automated drug delivery system for focal epilepsy. Epilepsy Res. 2000, 39, 103–114. [Google Scholar] [CrossRef]
- Salam, M.T.; Mirzaei, M.; Ly, M.S.; Nguyen, D.K.; Sawan, M. An implantable closedloop asynchronous drug delivery system for the treatment of refractory epilepsy. IEEE Trans. Neural. Syst. Rehabil. Eng. 2012, 20, 432–442. [Google Scholar] [CrossRef]
- Ludvig, N.; Medveczky, G.; Rizzolo, R.; Tang, H.M.; Baptiste, S.L.; Doyle, W.K.; Devinsky, O.; Carlson, C.; French, J.A.; Kral, J.G.; et al. An implantable triple-function device for local drug delivery, cerebrospinal fluid removal and EEG recording in the cranial subdural/subarachnoid space of primates. J. Neurosci. Methods 2012, 203, 275–283. [Google Scholar] [CrossRef]
- Bennewitz, M.F.; Saltzman, W.M. Nanotechnology for delivery of drugs to the brain for epilepsy. Neurotherapeutics 2009, 6, 323–336. [Google Scholar] [CrossRef]
- Löscher, W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure 2011, 20, 359–368. [Google Scholar] [CrossRef] [Green Version]
- Löscher, W. Fit for purpose application of currently existing animal models in the discovery of novel epilepsy therapies. Epilepsy Res. 2016, 126, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. Preclinical assessment of proconvulsant drug activity and its relevance for predicting adverse events in humans. Eur. J. Pharm. 2009, 610, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Goddard, G.V.; McIntyre, D.C.; Leech, C.K. A permanent change in brain function resulting from daily electrical stimulation. Exp. Neurol. 1969, 25, 295–330. [Google Scholar] [CrossRef]
- Stables, J.P.; Bertram, E.; Dudek, F.E.; Holmes, G.; Mathern, G.; Pitkanen, A.; White, H.S. Therapy discovery for pharmacoresistant epilepsy and for disease-modifying therapeutics: Summary of the NIH/NINDS/AES models II workshop. Epilepsia 2003, 44, 1472–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, M.; Racine, R.J.; McIntyre, D.C. Kindling: Basic mechanisms and clinical validity. Electroencephalogr. Clin. Neurophysiol. 1990, 76, 459–472. [Google Scholar] [CrossRef]
- Gernert, M.; Löscher, W. Lack of robust anticonvulsant effects of muscimol microinfusions in the anterior substantia nigra of kindled rats. Eur. J. Pharm. 2001, 432, 35–41. [Google Scholar] [CrossRef]
- Pollack, G.M.; Shen, D.D. A timed intravenous pentylenetetrazol infusion seizure model for quantitating the anticonvulsant effect of valproic acid in the rat. J. Pharm. Methods 1985, 13, 135–146. [Google Scholar] [CrossRef]
- Green, A.R.; Murray, T.K. A simple intravenous infusion method in rodents for determining the potency of anticonvulsants acting through GABAergic mechanisms. J. Pharm. Pharm. 1989, 41, 879–880. [Google Scholar] [CrossRef]
- Orloff, M.J.; Williams, H.L.; Pfeiffer, C.C. Timed intravenous infusion of metrazol and strychnine for testing anticonvulsant drugs. Proc. Soc. Exp. Biol. Med. 1949, 70, 254–257. [Google Scholar] [CrossRef]
- Löscher, W.; Jäckel, R.; Czuczwar, S.J. Is amygdala kindling in rats a model for drug-resistant partial epilepsy? Exp. Neurol. 1986, 93, 211–226. [Google Scholar] [CrossRef]
- Nilsen, K.E.; Walker, M.C.; Cock, H.R. Characterization of the tetanus toxin model of refractory focal neocortical epilepsy in the rat. Epilepsia 2005, 46, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Pfeffer, J.L.; Gururangan, S.; Lester, T.; Lim, D.A.; Shaywitz, A.J.; Westphal, M.; Slavc, I. Intracerebroventricular Delivery as a Safe, Long-Term Route of Drug Administration. Pediatr. Neurol. 2017, 67, 23–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croucher, M.J.; Meldrum, B.S.; Krogsgaard-Larsen, P. Anticonvulsant activity of GABA uptake inhibitors and their prodrugs following central or systemic administration. Eur. J. Pharm. 1983, 89, 217–228. [Google Scholar] [CrossRef]
- Gonsalves, S.F.; Twitchell, B.; Harbaugh, R.E.; Krogsgaard-Larsen, P.; Schousboe, A. Anticonvulsant activity of intracerebroventricularly administered glial GABA uptake inhibitors and other GABAmimetics in chemical seizure models. Epilepsy Res. 1989, 4, 34–41. [Google Scholar] [CrossRef]
- Gonzalez-Darder, J.M.; Garcia-Teno, M. Anticonvulsant effect of intraventricular antiepileptic drugs. Experimental study. Neurol. Res. 1995, 17, 190–192. [Google Scholar] [CrossRef]
- Barcia, J.A.; Rubio, P.; Alos, M.; Serralta, A.; Belda, V. Anticonvulsant and neurotoxic effects of intracerebroventricular injection of phenytoin, phenobarbital and carbamazepine in an amygdala-kindling model of epilepsy in the rat. Epilepsy Res. 1999, 33, 159–167. [Google Scholar] [CrossRef]
- Walrave, L.; Maes, K.; Coppens, J.; Bentea, E.; Van Eeckhaut, A.; Massie, A.; Van Liefferinge, J.; Smolders, I. Validation of the 6 Hz refractory seizure mouse model for intracerebroventricularly administered compounds. Epilepsy Res. 2015, 115, 67–72. [Google Scholar] [CrossRef]
- Czlonkowska, A.I.; Krzascik, P.; Sienkiewicz-Jarosz, H.; Siemiatkowski, M.; Szyndler, J.; Maciejak, P.; Bidzinski, A.; Plaznik, A. Tolerance to the anticonvulsant activity of midazolam and allopregnanolone in a model of picrotoxin seizures. Eur. J. Pharm. 2001, 425, 121–127. [Google Scholar] [CrossRef]
- Harada, C.; Hirai, T.; Fujii, Y.; Harusawa, S.; Kurihara, T.; Kamei, C. Intracerebroventricular administration of histamine H3 receptor antagonists decreases seizures in rat models of epilepsia. Methods Find Exp. Clin. Pharm. 2004, 26, 263–270. [Google Scholar]
- Kordi Jaz, E.; Moghimi, A.; Fereidoni, M.; Asadi, S.; Shamsizadeh, A.; Roohbakhsh, A. SB-334867, an orexin receptor 1 antagonist, decreased seizure and anxiety in pentylenetetrazol-kindled rats. Fundam. Clin. Pharm. 2017, 31, 201–207. [Google Scholar] [CrossRef]
- Asadi, S.; Roohbakhsh, A.; Shamsizadeh, A.; Fereidoni, M.; Kordijaz, E.; Moghimi, A. The effect of intracerebroventricular administration of orexin receptor type 2 antagonist on pentylenetetrazol-induced kindled seizures and anxiety in rats. BMC Neurosci. 2018, 19, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bostanci, M.O.; Bagirici, F. Anticonvulsive effects of quinine on penicillin-induced epileptiform activity: An in vivo study. Seizure 2007, 16, 166–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bostanci, M.O.; Bagirici, F. Anticonvulsive effects of carbenoxolone on penicillin-induced epileptiform activity: An in vivo study. Neuropharmacology 2007, 52, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Noga, D.A.; Brandao, L.E.; Cagni, F.C.; Silva, D.; de Azevedo, D.L.; Araujo, A.; Dos Santos, W.F.; Miranda, A.; da Silva, R.H.; Ribeiro, A.M. Anticonvulsant Effects of Fractions Isolated from Dinoponera quadriceps (Kempt) Ant Venom (Formicidae: Ponerinae). Toxins 2016, 9, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleischhack, G.; Jaehde, U.; Bode, U. Pharmacokinetics following intraventricular administration of chemotherapy in patients with neoplastic meningitis. Clin. Pharm. 2005, 44, 1–31. [Google Scholar] [CrossRef]
- Löscher, W.; Fisher, J.E.; Nau, H.; Hönack, D. Valproic acid in amygdala-kindled rats: Alterations in anticonvulsant efficacy, adverse effects and drug and metabolite levels in various brain regions during chronic treatment. J. Pharm. Exp. 1989, 250, 1067–1078. [Google Scholar]
- Martinez, R.; Vaquero, J.; De La Morena, L.V.; Tendillo, F.; Aragones, P. Toxicology and kinetics of long-term intraventricular infusion of phenytoin and valproic acid in pigs: Experimental study. Acta Neurochir. Suppl. (Wien) 1991, 52, 3–4. [Google Scholar] [CrossRef]
- Oommen, J.; Kraus, A.C.; Fisher, R.S. Intraventricular administration of gabapentin in the rat increases flurothyl seizure threshold. Neurosci. Lett. 2007, 417, 308–311. [Google Scholar] [CrossRef]
- Boison, D.; Scheurer, L.; Tseng, J.L.; Aebischer, P.; Mohler, H. Seizure suppression in kindled rats by intraventricular grafting of an adenosine releasing synthetic polymer. Exp. Neurol. 1999, 160, 164–174. [Google Scholar] [CrossRef]
- Study to Assess Intracerebroventricular (ICV) Delivery of CT-010 in Subjects with Focal Seizures, with Temporal Lobe Onset with or without Secondary Generalization. Available online: https://clinicaltrials.gov/ct2/show/NCT04153175 (accessed on 8 September 2020).
- Halliday, A.J.; Campbell, T.E.; Nelson, T.S.; McLean, K.J.; Wallace, G.G.; Cook, M.J. Levetiracetam-loaded biodegradable polymer implants in the tetanus toxin model of temporal lobe epilepsy in rats. J. Clin. Neurosci. 2013, 20, 148–152. [Google Scholar] [CrossRef] [Green Version]
- Ludvig, N.; Switzer, R.C.; Tang, H.M.; Kuzniecky, R.I. Autoradiographic evidence for the transmeningeal diffusion of muscimol into the neocortex in rats. Brain Res. 2012, 1441, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.C. Anticonvulsant effects of muscimol. Neurology 1980, 30, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Eder, H.G.; Jones, D.B.; Fisher, R.S. Local perfusion of diazepam attenuates interictal and ictal events in the bicuculline model of epilepsy in rats. Epilepsia 1997, 38, 516–521. [Google Scholar] [CrossRef] [Green Version]
- Ludvig, N.; Kuzniecky, R.I.; Baptiste, S.L.; John, J.E.; von Gizycki, H.; Doyle, W.K.; Devinsky, O. Epidural pentobarbital delivery can prevent locally induced neocortical seizures in rats: The prospect of transmeningeal pharmacotherapy for intractable focal epilepsy. Epilepsia 2006, 47, 1792–1802. [Google Scholar] [CrossRef] [PubMed]
- Ludvig, N.; Baptiste, S.L.; Tang, H.M.; Medveczky, G.; von Gizycki, H.; Charchaflieh, J.; Devinsky, O.; Kuzniecky, R.I. Localized transmeningeal muscimol prevents neocortical seizures in rats and nonhuman primates: Therapeutic implications. Epilepsia 2009, 50, 678–693. [Google Scholar] [CrossRef] [PubMed]
- Baptiste, S.L.; Tang, H.M.; Kuzniecky, R.I.; Devinsky, O.; French, J.A.; Ludvig, N. Comparison of the antiepileptic properties of transmeningeally delivered muscimol, lidocaine, midazolam, pentobarbital and GABA, in rats. Neurosci. Lett. 2010, 469, 421–424. [Google Scholar] [CrossRef] [PubMed]
- John, J.E.; Baptiste, S.L.; Sheffield, L.G.; von Gizycki, H.; Kuzniecky, R.I.; Devinsky, O.; Ludvig, N. Transmeningeal delivery of GABA to control neocortical seizures in rats. Epilepsy Res. 2007, 75, 10–17. [Google Scholar] [CrossRef]
- Fukuda, H.; Brailowsky, S.; Menini, C.; Silva-Barrat, C.; Riche, D.; Naquet, R. Anticonvulsant effect of intracortical, chronic infusion of GABA in kindled rats: Focal seizures upon withdrawal. Exp. Neurol. 1987, 98, 120–129. [Google Scholar] [CrossRef]
- Casasola, C.; Bargas, J.; Arias-Montano, J.A.; Calixto, E.; Montiel, T.; Galarraga, E.; Brailowsky, S. Hippocampal hyperexcitability induced by GABA withdrawal is due to down-regulation of GABA(A) receptors. Epilepsy Res. 2001, 47, 257–271. [Google Scholar] [CrossRef]
- Ludvig, N.; Tang, H.M.; Artan, N.S.; Mirowski, P.; Medveczky, G.; Baptiste, S.L.; Darisi, S.; Kuzniecky, R.I.; Devinsky, O.; French, J.A. Transmeningeal muscimol can prevent focal EEG seizures in the rat neocortex without stopping multineuronal activity in the treated area. Brain Res. 2011, 1385, 182–191. [Google Scholar] [CrossRef]
- Ludvig, N.; Tang, H.M.; Baptiste, S.L.; Medveczky, G.; Vaynberg, J.K.; Vazquez-DeRose, J.; Stefanov, D.G.; Devinsky, O.; French, J.A.; Carlson, C.; et al. Long-term behavioral, electrophysiological, and neurochemical monitoring of the safety of an experimental antiepileptic implant, the muscimol-delivering Subdural Pharmacotherapy Device in monkeys Laboratory investigation. J. Neurosurg. 2012, 117, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Ludvig, N.; Tang, H.M.; Baptiste, S.L.; Stefanov, D.G.; Kral, J.G. Spatial memory in nonhuman primates implanted with the subdural pharmacotherapy device. Behav. Brain Res. 2015, 286, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Kelso, A.R.C.; Nilsen, K.E.; Cock, H.R. Focal delivery of standard antiepileptic drugs in the tetanus toxin model of epilepsy in rats. J. Neurol. 2005, 252, ii/16. [Google Scholar]
- Smolders, I.; Khan, G.M.; Lindekens, H.; Prikken, S.; Marvin, C.A.; Manil, J.; Ebinger, G.; Michotte, Y. Effectiveness of vigabatrin against focally evoked pilocarpine-induced seizures and concomitant changes in extracellular hippocampal and cerebellar glutamate, gamma-aminobutyric acid and dopamine levels, a microdialysis-electrocorticography study in freely moving rats. J. Pharm. Exp. 1997, 283, 1239–1248. [Google Scholar]
- Eder, H.G.; Stein, A.; Fisher, R.S. Interictal and ictal activity in the rat cobalt/pilocarpine model of epilepsy decreased by local perfusion of diazepam. Epilepsy Res. 1997, 29, 17–24. [Google Scholar] [CrossRef]
- Piredda, S.; Gale, K. A crucial epileptogenic site in the deep prepiriform cortex. Nature 1985, 317, 623–625. [Google Scholar] [CrossRef]
- Smith, D.C.; Krahl, S.E.; Browning, R.A.; Barea, E.J. Rapid cessation of focally induced generalized seizures in rats through microinfusion of lidocaine hydrochloride into the focus. Epilepsia 1993, 34, 43–53. [Google Scholar] [CrossRef]
- Uemura, S.; Ienaga, K.; Higashiura, K.; Kimura, H. Effects of intraamygdaloid injection of taurine and valyltaurine on amygdaloid kindled seizure in rats. Jpn. J. Psychiatry Neurol. 1991, 45, 383–385. [Google Scholar] [CrossRef]
- Attwell, P.J.; Koumentaki, A.; Abdul-Ghani, A.S.; Croucher, M.J.; Bradford, H.F. Specific group II metabotropic glutamate receptor activation inhibits the development of kindled epilepsy in rats. Brain Res. 1998, 787, 286–291. [Google Scholar] [CrossRef]
- Croucher, M.J.; Bradford, H.F.; Sunter, D.C.; Watkins, J.C. Inhibition of the development of electrical kindling of the prepyriform cortex by daily focal injections of excitatory amino acid antagonists. Eur. J. Pharm. 1988, 152, 29–38. [Google Scholar] [CrossRef]
- Holmes, K.H.; Bilkey, D.K.; Laverty, R. The infusion of an NMDA antagonist into perirhinal cortex suppresses amygdala-kindled seizures. Brain Res. 1992, 587, 285–290. [Google Scholar] [CrossRef]
- Alam, A.M.; Starr, M.S. Dopaminergic modulation of pilocarpine-induced motor seizures in the rat: The role of hippocampal D2 receptors. Neuroscience 1993, 53, 425–431. [Google Scholar] [CrossRef]
- Pourgholami, M.H.; Rostampour, M.; Mirnajafi-Zadeh, J.; Palizvan, M.R. Intra-amygdala infusion of 2-chloroadenosine suppresses amygdala-kindled seizures. Brain Res. 1997, 775, 37–42. [Google Scholar] [CrossRef]
- Franklin, P.H.; Zhang, G.; Tripp, E.D.; Murray, T.F. Adenosine A1 receptor activation mediates suppression of (-) bicuculline methiodide-induced seizures in rat prepiriform cortex. J. Pharm. Exp. 1989, 251, 1229–1236. [Google Scholar]
- Anschel, D.J.; Ortega, E.L.; Kraus, A.C.; Fisher, R.S. Focally injected adenosine prevents seizures in the rat. Exp. Neurol. 2004, 190, 544–547. [Google Scholar] [CrossRef]
- Bagirici, F.; Bostanci, M.O. Anticonvulsive effects of nimodipine on penicillin-induced epileptiform activity. Acta Neurobiol. Exp. (Wars) 2006, 66, 123–128. [Google Scholar]
- Nilsen, K.E.; Kelso, A.R.; Cock, H.R. Antiepileptic effect of gap-junction blockers in a rat model of refractory focal cortical epilepsy. Epilepsia 2006, 47, 1169–1175. [Google Scholar] [CrossRef]
- Sayyah, M.; Rezaie, M.; Haghighi, S.; Amanzadeh, A. Intra-amygdala all-trans retinoic acid inhibits amygdala-kindled seizures in rats. Epilepsy Res. 2007, 75, 97–103. [Google Scholar] [CrossRef]
- Costantin, L.; Bozzi, Y.; Richichi, C.; Viegi, A.; Antonucci, F.; Funicello, M.; Gobbi, M.; Mennini, T.; Rossetto, O.; Montecucco, C.; et al. Antiepileptic effects of botulinum neurotoxin E. J. Neurosci. 2005, 25, 1943–1951. [Google Scholar] [CrossRef] [Green Version]
- Erfanparast, A.; Tamaddonfard, E.; Henareh-Chareh, F. Intra-hippocampal microinjection of oxytocin produced antiepileptic effect on the pentylenetetrazol-induced epilepsy in rats. Pharm. Rep. 2017, 69, 757–763. [Google Scholar] [CrossRef]
- Ataie, Z.; Babri, S.; Ghahramanian Golzar, M.; Ebrahimi, H.; Mirzaie, F.; Mohaddes, G. GABAB Receptor Blockade Prevents Antiepileptic Action of Ghrelin in the Rat Hippocampus. Adv. Pharm. Bull. 2013, 3, 353–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goudarzi, E.; Elahdadi Salmani, M.; Lashkarbolouki, T.; Goudarzi, I. Hippocampal orexin receptors inactivation reduces PTZ induced seizures of male rats. Pharmacol. Biochem. Behav. 2015, 130, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, A.S.; Attwell, P.J.; Bradford, H.F. The anti-epileptic effect of 3-aminopropylarsonate on electrically-kindled and N-methyl-D-aspartate-kindled amygdala. Brain Res. 1996, 742, 305–312. [Google Scholar] [CrossRef]
- Abdul-Ghani, A.S.; Attwell, P.J.; Bradford, H.F. The effect of 2-amino-3-arsonopropionate and 2-amino-4-arsonobutyrate on the development and maintenance of amygdala kindled seizures. Int. J. Neurosci. 1998, 96, 255–267. [Google Scholar] [CrossRef]
- Abdul-Ghani, A.S.; Attwell, P.J.; Singh Kent, N.; Bradford, H.F.; Croucher, M.J.; Jane, D.E. Anti-epileptogenic and anticonvulsant activity of L-2-amino-4-phosphonobutyrate, a presynaptic glutamate receptor agonist. Brain Res. 1997, 755, 202–212. [Google Scholar] [CrossRef]
- Abdul-Ghani, A.S.; Attwell, P.J.; Bradford, H.F. The protective effect of 2-chloroadenosine against the development of amygdala kindling and on amygdala-kindled seizures. Eur. J. Pharm. 1997, 326, 7–14. [Google Scholar] [CrossRef]
- Mirnajafi-Zadeh, J.; Mortazavi, M.; Fathollahi, Y.; Alasvand Zarasvand, M.; Reza Palizvan, M. Effect of transient hippocampal inhibition on amygdaloid kindled seizures and amygdaloid kindling rate. Brain Res. 2002, 954, 220–226. [Google Scholar] [CrossRef]
- Applegate, C.D.; Burchfiel, J.L. Microinjections of Gaba Agonists into the Amygdala Complex Attenuates Kindled Seizure Expression in the Rat. Exp. Neurol. 1988, 102, 185–189. [Google Scholar] [CrossRef]
- Meldrum, B.; Millan, M.; Patel, S.; de Sarro, G. Anti-epileptic effects of focal micro-injection of excitatory amino acid antagonists. J. Neural. Transm. 1988, 72, 191–200. [Google Scholar] [CrossRef]
- Piredda, S.; Pavlick, M.; Gale, K. Anticonvulsant effects of GABA elevation in the deep prepiriform cortex. Epilepsy Res. 1987, 1, 102–106. [Google Scholar] [CrossRef]
- Schwabe, K.; Ebert, U.; Löscher, W. The central piriform cortex: Anatomical connections and anticonvulsant effect of GABA elevation in the kindling model. Neuroscience 2004, 126, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.R.; Phillips, I.; de Beaurepaire, R. gamma-Vinyl GABA in endopiriform area suppresses kindled amygdala seizures. Epilepsia 1988, 29, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Jackel, R.; Muller, F. Anticonvulsant and proconvulsant effects of inhibitors of GABA degradation in the amygdala-kindling model. Eur. J. Pharm. 1989, 163, 1–14. [Google Scholar] [CrossRef]
- Löscher, W.; Ebert, U. The role of the piriform cortex in kindling. Prog. Neurobiol. 1996, 50, 427–481. [Google Scholar] [CrossRef]
- Gernert, M.; Bloms-Funke, P.; Ebert, U.; Löscher, W. Kindling causes persistent in vivo changes in firing rates and glutamate sensitivity of central piriform cortex neurons in rats. Neuroscience 2000, 99, 217–227. [Google Scholar] [CrossRef]
- Lehmann, H.; Ebert, U.; Löscher, W. Amygdala-kindling induces a lasting reduction of GABA-immunoreactive neurons in a discrete area of the ipsilateral piriform cortex. Synapse 1998, 29, 299–309. [Google Scholar] [CrossRef]
- Laufs, H.; Richardson, M.P.; Salek-Haddadi, A.; Vollmar, C.; Duncan, J.S.; Gale, K.; Lemieux, L.; Löscher, W.; Koepp, M.J. Converging PET and fMRI evidence for a common area involved in human focal epilepsies. Neurology 2011, 77, 904–910. [Google Scholar] [CrossRef] [Green Version]
- Kohane, D.S.; Holmes, G.L.; Chau, Y.; Zurakowski, D.; Langer, R.; Cha, B.H. Effectiveness of muscimol-containing microparticles against pilocarpine-induced focal seizures. Epilepsia 2002, 43, 1462–1468. [Google Scholar] [CrossRef]
- Kokaia, M.; Aebischer, P.; Elmer, E.; Bengzon, J.; Kalen, P.; Kokaia, Z.; Lindvall, O. Seizure suppression in kindling epilepsy by intracerebral implants of GABA- but not by noradrenaline-releasing polymer matrices. Exp. Brain Res. 1994, 100, 385–394. [Google Scholar] [CrossRef]
- Jiang, J.L.; Yue, Z.; Bauquier, S.H.; Lai, A.; Chen, Y.; McLean, K.J.; Halliday, A.J.; Sui, Y.; Moulton, S.; Wallace, G.G.; et al. Injectable phenytoin loaded polymeric microspheres for the control of temporal lobe epilepsy in rats. Restor. Neurol. Neurosci. 2015, 33, 823–834. [Google Scholar] [CrossRef] [Green Version]
- Tamargo, R.J.; Rossell, L.A.; Kossoff, E.H.; Tyler, B.M.; Ewend, M.G.; Aryanpur, J.J. The intracerebral administration of phenytoin using controlled-release polymers reduces experimental seizures in rats. Epilepsy Res. 2002, 48, 145–155. [Google Scholar] [CrossRef]
- Lopez, T.; Ortiz, E.; Quintana, P.; Gonzalez, R.D. A nanostructured titania bioceramic implantable device capable of drug delivery to the temporal lobe of the brain. Colloids Surf. A 2007, 300, 3–10. [Google Scholar] [CrossRef]
- Altenmüller, D.M.; Hebel, J.M.; Rassner, M.P.; Volz, S.; Freiman, T.M.; Feuerstein, T.J.; Zentner, J. Locally applied valproate enhances survival in rats after neocortical treatment with tetanus toxin and cobalt chloride. BioMed Res. Int. 2013, 2013, 497485. [Google Scholar] [CrossRef]
- Rassner, M.P.; Hebel, J.M.; Altenmüller, D.M.; Volz, S.; Herrmann, L.S.; Feuerstein, T.J.; Freiman, T.M. Reduction of epileptiform activity through local valproate-implants in a rat neocortical epilepsy model. Seizure 2015, 30, 6–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubek, M.J.; Liang, D.; Byrd, K.E.; Domb, A.J. Prolonged seizure suppression by a single implantable polymeric-TRH microdisk preparation. Brain Res. 1998, 809, 189–197. [Google Scholar] [CrossRef]
- Wilz, A.; Pritchard, E.M.; Li, T.; Lan, J.Q.; Kaplan, D.L.; Boison, D. Silk polymer-based adenosine release: Therapeutic potential for epilepsy. Biomaterials 2008, 29, 3609–3616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szybala, C.; Pritchard, E.M.; Lusardi, T.A.; Li, T.; Wilz, A.; Kaplan, D.L.; Boison, D. Antiepileptic effects of silk-polymer based adenosine release in kindled rats. Exp. Neurol. 2009, 219, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Gasior, M.; White, N.A.; Rogawski, M.A. Prolonged attenuation of amygdala-kindled seizure measures in rats by convection-enhanced delivery of the N-type calcium channel antagonists omega-conotoxin GVIA and omega-conotoxin MVIIA. J. Pharm. Exp. 2007, 323, 458–468. [Google Scholar] [CrossRef] [Green Version]
- Gasior, M.; Tang, R.; Rogawski, M.A. Long-lasting attenuation of amygdala-kindled seizures after convection-enhanced delivery of botulinum neurotoxins A and B into the amygdala in rats. J. Pharm. Exp. 2013, 346, 528–534. [Google Scholar] [CrossRef] [Green Version]
- Bröer, S.; Zolkowska, D.; Gernert, M.; Rogawski, M.A. Proconvulsant actions of intrahippocampal botulinum neurotoxin B in the rat. Neuroscience 2013, 252, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Mangubat, E.Z.; Kellogg, R.G.; Harris, T.J., Jr.; Rossi, M.A. On-demand pulsatile intracerebral delivery of carisbamate with closed-loop direct neurostimulation therapy in an electrically induced self-sustained focal-onset epilepsy rat model. J. Neurosurg. 2015, 122, 1283–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiss, J.D.; Walbridge, S.; Argersinger, D.P.; Hong, C.S.; Ray-Chaudhury, A.; Lonser, R.R.; Elias, W.J.; Zaghloul, K.A. Convection-Enhanced Delivery of Muscimol Into the Bilateral Subthalamic Nuclei of Nonhuman Primates. Neurosurgery 2019, 84, E420–E429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiss, J.D.; Walbridge, S.; Asthagiri, A.R.; Lonser, R.R. Image-guided convection-enhanced delivery of muscimol to the primate brain. J. Neurosurg. 2010, 112, 790–795. [Google Scholar] [CrossRef]
- Heiss, J.D.; Walbridge, S.; Morrison, P.; Hampton, R.R.; Sato, S.; Vortmeyer, A.; Butman, J.A.; O’Malley, J.; Vidwan, P.; Dedrick, R.L.; et al. Local distribution and toxicity of prolonged hippocampal infusion of muscimol. J. Neurosurg. 2005, 103, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Gale, K.; Proctor, M.; Velísková, J.; Nehlig, A. Basal ganglia and brainstem anatomy and physiology. In Epilepsy: A Comprehensive Textbook, 2nd ed.; Engel, J.J., Pedley, T.A., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; pp. 367–384. [Google Scholar]
- Rektor, I.; Kuba, R.; Brazdil, M.; Chrastina, J. Do the basal ganglia inhibit seizure activity in temporal lobe epilepsy? Epilepsy Behav. 2012, 25, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Aupy, J.; Wendling, F.; Taylor, K.; Bulacio, J.; Gonzalez-Martinez, J.; Chauvel, P. Cortico-striatal synchronization in human focal seizures. Brain 2019, 142, 1282–1295. [Google Scholar] [CrossRef] [PubMed]
- DeLong, M.; Wichmann, T. Changing views of basal ganglia circuits and circuit disorders. Clin. EEG Neurosci. 2010, 41, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Nelson, A.B.; Kreitzer, A.C. Reassessing models of basal ganglia function and dysfunction. Annu. Rev. Neurosci. 2014, 37, 117–135. [Google Scholar] [CrossRef] [Green Version]
- Soper, C.; Wicker, E.; Kulick, C.V.; N’Gouemo, P.; Forcelli, P.A. Optogenetic activation of superior colliculus neurons suppresses seizures originating in diverse brain networks. Neurobiol. Dis. 2016, 87, 102–115. [Google Scholar] [CrossRef] [Green Version]
- Redgrave, P.; Coizet, V.; Comoli, E.; McHaffie, J.G.; Leriche, M.; Vautrelle, N.; Hayes, L.M.; Overton, P. Interactions between the Midbrain Superior Colliculus and the Basal Ganglia. Front. Neuroanat. 2010, 4, 132. [Google Scholar] [CrossRef] [Green Version]
- Gale, K.; Pazos, A.; Maggio, R.; Japikse, K.; Pritchard, P. Blockade of GABA receptors in superior colliculus protects against focally evoked limbic motor seizures. Brain Res. 1993, 603, 279–283. [Google Scholar] [CrossRef]
- Hikosaka, O.; Sesack, S.R.; Lecourtier, L.; Shepard, P.D. Habenula: Crossroad between the basal ganglia and the limbic system. J. Neurosci. 2008, 28, 11825–11829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kücker, S.; Töllner, K.; Piechotta, M.; Gernert, M. Kindling as a model of temporal lobe epilepsy induces bilateral changes in spontaneous striatal activity. Neurobiol. Dis. 2010, 37, 661–672. [Google Scholar] [CrossRef]
- Gernert, M.; Fedrowitz, M.; Wlaz, P.; Löscher, W. Subregional changes in discharge rate, pattern, and drug sensitivity of putative GABAergic nigral neurons in the kindling model of epilepsy. Eur. J. Neurosci. 2004, 20, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Töllner, K.; Wolf, S.; Löscher, W.; Gernert, M. The anticonvulsant response to valproate in kindled rats is correlated with its effect on neuronal firing in the substantia nigra pars reticulata: A new mechanism of pharmacoresistance. J. Neurosci. 2011, 31, 16423–16434. [Google Scholar] [CrossRef]
- Gernert, M.; Fedrowitz, M.; Wlaz, P.; Löscher, W. Are changes in the discharge rate of GABAergic nigral neurons in the rat kindling model of epilepsy attributable to changes in subthalamic neuronal activity? Epilepsia 2002, 43 (Suppl. S8), 81–82. [Google Scholar]
- Nolte, M.W.; Löscher, W.; Gernert, M. Pedunculopontine neurons are involved in network changes in the kindling model of temporal lobe epilepsy. Neurobiol. Dis. 2006, 23, 206–218. [Google Scholar] [CrossRef]
- Löscher, W.; Schwark, W.S. Evidence for impaired GABAergic activity in the substantia nigra of amygdaloid kindled rats. Brain Res. 1985, 339, 146–150. [Google Scholar] [CrossRef]
- Löscher, W.; Schwark, W.S. Further evidence for abnormal GABAergic circuits in amygdala-kindled rats. Brain Res. 1987, 420, 385–390. [Google Scholar] [CrossRef]
- Turski, W.A.; Cavalheiro, E.A.; Schwarz, M.; Czuczwar, S.J.; Kleinrok, Z.; Turski, L. Limbic seizures produced by pilocarpine in rats: Behavioural, electroencephalographic and neuropathological study. Behav. Brain Res. 1983, 9, 315–335. [Google Scholar] [CrossRef]
- Freichel, C.; Ebert, U.; Potschka, H.; Löscher, W. Amygdala-kindling does not induce a persistent loss of GABA neurons in the substantia nigra pars reticulata of rats. Brain Res. 2004, 1025, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, H.; Varghese, G.I.; Purcaro, M.J.; Motelow, J.E.; Enev, M.; McNally, K.A.; Levin, A.R.; Hirsch, L.J.; Tikofsky, R.; Zubal, I.G.; et al. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain 2009, 132, 999–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Li, H.; Zhu, L.; Yu, X.; Jin, B.; Chen, C.; Wang, S.; Ding, M.; Zhang, M.; Chen, Z.; et al. Localized shape abnormalities in the thalamus and pallidum are associated with secondarily generalized seizures in mesial temporal lobe epilepsy. Epilepsy Behav. 2017, 70, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, H.; McNally, K.A.; Vanderhill, S.D.; Paige, A.L.; Chung, R.; Davis, K.; Norden, A.D.; Stokking, R.; Studholme, C.; Novotny, E.J., Jr.; et al. Positive and negative network correlations in temporal lobe epilepsy. Cereb. Cortex 2004, 14, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, H.P.; Kuzniecky, R.I.; Vives, K.; Devinsky, O.; Pacia, S.; Luciano, D.; Vasquez, B.; Haut, S.; Spencer, D.D.; Pan, J.W. A subcortical network of dysfunction in TLE measured by magnetic resonance spectroscopy. Neurology 2007, 69, 2256–2265. [Google Scholar] [CrossRef]
- Gale, K.; Iadarola, M.J. Seizure protection and increased nerve-terminal GABA: Delayed effects of GABA transaminase inhibition. Science 1980, 208, 288–291. [Google Scholar] [CrossRef]
- Gale, K.; Iadarola, M. Drug-induced elevation of GABA after intracerebral microinjection: Site of anticonvulsant action. Eur. J. Pharm. 1980, 68, 233–235. [Google Scholar] [CrossRef]
- Iadarola, M.J.; Gale, K. Substantia nigra: Site of anticonvulsant activity mediated by gamma-aminobutyric acid. Science 1982, 218, 1237–1240. [Google Scholar] [CrossRef]
- Depaulis, A.; Vergnes, M.; Marescaux, C. Endogenous control of epilepsy: The nigral inhibitory system. Prog. Neurobiol. 1994, 42, 33–52. [Google Scholar] [CrossRef]
- Deransart, C.; Le-Pham, B.T.; Hirsch, E.; Marescaux, C.; Depaulis, A. Inhibition of the substantia nigra suppresses absences and clonic seizures in audiogenic rats, but not tonic seizures: Evidence for seizure specificity of the nigral control. Neuroscience 2001, 105, 203–211. [Google Scholar] [CrossRef]
- Shehab, S.A.; Ljubisavljevic, M.; Al-Halhali, F.; Al-Awadhi, A.; Madathil, M.; Abdul-Kareem, A.; Redgrave, P. Experimental manipulations of the subthalamic nucleus fail to suppress tonic seizures in the electroshock model of epilepsy. Exp. Brain Res. 2006, 173, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Turski, L.; Cavalheiro, E.A.; Schwarz, M.; Turski, W.A.; De Moraes Mello, L.E.; Bortolotto, Z.A.; Klockgether, T.; Sontag, K.H. Susceptibility to seizures produced by pilocarpine in rats after microinjection of isoniazid or gamma-vinyl-GABA into the substantia nigra. Brain Res. 1986, 370, 294–309. [Google Scholar] [CrossRef]
- Turski, L.; Cavalheiro, E.A.; Turski, W.A.; Meldrum, B.S. Excitatory neurotransmission within substantia nigra pars reticulata regulates threshold for seizures produced by pilocarpine in rats: Effects of intranigral 2-amino-7-phosphonoheptanoate and N-methyl-D-aspartate. Neuroscience 1986, 18, 61–77. [Google Scholar] [CrossRef]
- DeSarro, G.; Patel, S.; Meldrum, B.S. Anticonvulsant action of a kainate antagonist gamma-D-glutamyl aminomethylsulphonic acid injected focally into the substantia nigra and entopeduncular nucleus. Eur. J. Pharm. 1986, 132, 229–236. [Google Scholar] [CrossRef]
- Löscher, W.; Czuczwar, S.J.; Jackel, R.; Schwarz, M. Effect of microinjections of gamma-vinyl GABA or isoniazid into substantia nigra on the development of amygdala kindling in rats. Exp. Neurol. 1987, 95, 622–638. [Google Scholar] [CrossRef]
- Le Gal La Salle, G.; Kaijima, M.; Feldblum, S. Abortive amygdaloid kindled seizures following microinjection of gamma-vinyl-GABA in the vicinity of substantia nigra in rats. Neurosci. Lett. 1983, 36, 69–74. [Google Scholar] [CrossRef]
- McNamara, J.O.; Galloway, M.T.; Rigsbee, L.C.; Shin, C. Evidence implicating substantia nigra in regulation of kindled seizure threshold. J. Neurosci. 1984, 4, 2410–2417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deransart, C.; Le, B.T.; Marescaux, C.; Depaulis, A. Role of the subthalamo-nigral input in the control of amygdala-kindled seizures in the rat. Brain Res. 1998, 807, 78–83. [Google Scholar] [CrossRef]
- Maggio, R.; Gale, K. Seizures evoked from area tempestas are subject to control by GABA and glutamate receptors in substantia nigra. Exp. Neurol. 1989, 105, 184–188. [Google Scholar] [CrossRef]
- Xu, S.G.; Garant, D.S.; Sperber, E.F.; Moshé, S.L. Effects of substantia nigra gamma-vinyl-GABA infusions on flurothyl seizures in adult rats. Brain Res. 1991, 566, 108–114. [Google Scholar] [CrossRef]
- Bröer, S.; Backofen-Wehrhahn, B.; Bankstahl, M.; Gey, L.; Gernert, M.; Löscher, W. Vigabatrin for focal drug delivery in epilepsy: Bilateral microinfusion into the subthalamic nucleus is more effective than intranigral or systemic administration in a rat seizure model. Neurobiol. Dis. 2012, 46, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Garant, D.S.; Iadarola, M.J.; Gale, K. Substance P antagonists in substantia nigra are anticonvulsant. Brain Res. 1986, 382, 372–378. [Google Scholar] [CrossRef]
- Garant, D.S.; Gale, K. Infusion of opiates into substantia nigra protects against maximal electroshock seizures in rats. J. Pharm. Exp. 1985, 234, 45–48. [Google Scholar]
- Bonhaus, D.W.; Rigsbee, L.C.; McNamara, J.O. Intranigral dynorphin-1-13 suppresses kindled seizures by a naloxone-insensitive mechanism. Brain Res. 1987, 405, 358–363. [Google Scholar] [CrossRef]
- Pasini, A.; Tortorella, A.; Gale, K. Anticonvulsant effect of intranigral fluoxetine. Brain Res. 1992, 593, 287–290. [Google Scholar] [CrossRef]
- Klitgaard, H.; Matagne, A.; Grimee, R.; Vanneste-Goemaere, J.; Margineanu, D.G. Electrophysiological, neurochemical and regional effects of levetiracetam in the rat pilocarpine model of temporal lobe epilepsy. Seizure 2003, 12, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Meurs, A.; Clinckers, R.; Ebinger, G.; Michotte, Y.; Smolders, I. Substantia nigra is an anticonvulsant site of action of topiramate in the focal pilocarpine model of limbic seizures. Epilepsia 2006, 47, 1519–1535. [Google Scholar] [CrossRef]
- Waszczak, B.L.; Lee, E.K.; Walters, J.R. Effects of anticonvulsant drugs on substantia nigra pars reticulata neurons. J. Pharm. Exp. 1986, 239, 606–611. [Google Scholar]
- Turski, L.; Andrews, J.S.; Loschmann, P.A.; Bressler, K.; Bortolotto, Z.A.; Calderazzo-Filho, L.S.; Cavalheiro, E.A. Substantia nigra regulates action of antiepileptic drugs. Brain Res. 1990, 520, 232–239. [Google Scholar] [CrossRef]
- Giorgi, F.S.; Galanopoulou, A.S.; Moshe, S.L. Sex dimorphism in seizure-controlling networks. Neurobiol. Dis. 2014, 72 Pt B, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Shehab, S.; Simkins, M.; Dean, P.; Redgrave, P. Regional distribution of the anticonvulsant and behavioural effects of muscimol injected into the substantia nigra of rats. Eur. J. Neurosci. 1996, 8, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Velísková, J.; Moshé, S.L. Update on the role of substantia nigra pars reticulata in the regulation of seizures. Epilepsy Curr. 2006, 6, 83–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Rosenberg, H.C.; Tietz, E.I. Anticonvulsant actions and interaction of GABA agonists and a benzodiazepine in pars reticulata of substantia nigra. Epilepsy Res. 1991, 8, 11–20. [Google Scholar] [CrossRef]
- Ravizza, T.; Friedman, L.K.; Moshé, S.L.; Velísková, J. Sex differences in GABA(A)ergic system in rat substantia nigra pars reticulata. Int. J. Dev. Neurosci. 2003, 21, 245–254. [Google Scholar] [CrossRef]
- Turski, L.; Meldrum, B.S.; Cavalheiro, E.A.; Calderazzo-Filho, L.S.; Bortolotto, Z.A.; Ikonomidou-Turski, C.; Turski, W.A. Paradoxical anticonvulsant activity of the excitatory amino acid N-methyl-D-aspartate in the rat caudate-putamen. Proc. Natl. Acad. Sci. USA 1987, 84, 1689–1693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalheiro, E.A.; Turski, L. Intrastriatal N-methyl-D-aspartate prevents amygdala kindled seizures in rats. Brain Res. 1986, 377, 173–176. [Google Scholar] [CrossRef]
- Turski, L.; Cavalheiro, E.A.; Calderazzo-Filho, L.S.; Bortolotto, Z.A.; Klockgether, T.; Ikonomidou, C.; Turski, W.A. The basal ganglia, the deep prepyriform cortex, and seizure spread: Bicuculline is anticonvulsant in the rat striatum. Proc. Natl. Acad. Sci. USA 1989, 86, 1694–1697. [Google Scholar] [CrossRef] [Green Version]
- Cavalheiro, E.A.; Bortolotto, Z.A.; Turski, L. Microinjections of the gamma-aminobutyrate antagonist, bicuculline methiodide, into the caudate-putamen prevent amygdala-kindled seizures in rats. Brain Res. 1987, 411, 370–372. [Google Scholar] [CrossRef]
- Turski, L.; Cavalheiro, E.A.; Bortolotto, Z.A.; Ikonomidou-Turski, C.; Kleinrok, Z.; Turski, W.A. Dopamine-sensitive anticonvulsant site in the rat striatum. J. Neurosci. 1988, 8, 4027–4037. [Google Scholar] [CrossRef] [Green Version]
- Al-Tajir, G.; Starr, M.S. D-2 agonists protect rodents against pilocarpine-induced convulsions by stimulating D-2 receptors in the striatum, but not in the substantia nigra. Pharmacol. Biochem. Behav. 1991, 39, 109–113. [Google Scholar] [CrossRef]
- Wahnschaffe, U.; Löscher, W. Anticonvulsant effects of ipsilateral but not contralateral microinjections of the dopamine D2 agonist LY 171555 into the nucleus accumbens of amygdala-kindled rats. Brain Res. 1991, 553, 181–187. [Google Scholar] [CrossRef]
- Chen, L.; Chan, Y.S.; Yung, W.H. GABA-B receptor activation in the rat globus pallidus potently suppresses pentylenetetrazol-induced tonic seizures. J. Biomed Sci. 2004, 11, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Feger, J.; Robledo, P. The Effects of Activation or Inhibition of the Subthalamic Nucleus on the Metabolic and Electrophysiological Activities within the Pallidal Complex and Substantia Nigra in the Rat. Eur. J. Neurosci. 1991, 3, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Hamani, C.; Saint-Cyr, J.A.; Fraser, J.; Kaplitt, M.; Lozano, A.M. The subthalamic nucleus in the context of movement disorders. Brain 2004, 127, 4–20. [Google Scholar] [CrossRef] [Green Version]
- Bonnevie, T.; Zaghloul, K.A. The Subthalamic Nucleus: Unravelling New Roles and Mechanisms in the Control of Action. Neuroscientist 2019, 25, 48–64. [Google Scholar] [CrossRef]
- Deransart, C.; Marescaux, C.; Depaulis, A. Involvement of nigral glutamatergic inputs in the control of seizures in a genetic model of absence epilepsy in the rat. Neuroscience 1996, 71, 721–728. [Google Scholar] [CrossRef]
- Velísková, J.; Velsek, L.; Moshé, S.L. Subthalamic nucleus: A new anticonvulsant site in the brain. Neuroreport 1996, 7, 1786–1788. [Google Scholar] [CrossRef]
- Dybdal, D.; Gale, K. Postural and anticonvulsant effects of inhibition of the rat subthalamic nucleus. J. Neurosci. 2000, 20, 6728–6733. [Google Scholar] [CrossRef] [Green Version]
- Dybdal, D.; Forcelli, P.A.; Dubach, M.; Oppedisano, M.; Holmes, A.; Malkova, L.; Gale, K. Topography of dyskinesias and torticollis evoked by inhibition of substantia nigra pars reticulata. Mov. Disord. 2013, 28, 460–468. [Google Scholar] [CrossRef]
- Handreck, A.; Backofen-Wehrhahn, B.; Bröer, S.; Löscher, W.; Gernert, M. Anticonvulsant Effects by Bilateral and Unilateral Transplantation of GABA-Producing Cells Into the Subthalamic Nucleus in an Acute Seizure Model. Cell Transpl. 2014, 23, 111–132. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.H.; Luo, F.; Woodward, D.J.; McIntyre, D.C.; Chang, J.Y. Temporal sequence of ictal discharges propagation in the corticolimbic basal ganglia system during amygdala kindled seizures in freely moving rats. Epilepsy Res. 2007, 73, 85–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabardes, S.; Kahane, P.; Minotti, L.; Koudsie, A.; Hirsch, E.; Benabid, A.L. Deep brain stimulation in epilepsy with particular reference to the subthalamic nucleus. Epileptic Disord. 2002, 4 (Suppl. S3), S83–S93. [Google Scholar]
- Klinger, N.; Mittal, S. Deep brain stimulation for seizure control in drug-resistant epilepsy. Neurosurg. Focus 2018, 45, E4. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Lang, A.E.; Dostrovsky, J.O.; Pahapill, P.; Romas, J.; Saint-Cyr, J.; Hutchison, W.D.; Lozano, A.M. Lidocaine and muscimol microinjections in subthalamic nucleus reverse parkinsonian symptoms. Brain 2001, 124, 2105–2118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vertes, R.P.; Linley, S.B.; Hoover, W.B. Limbic circuitry of the midline thalamus. Neurosci. Biobehav. Rev. 2015, 54, 89–107. [Google Scholar] [CrossRef] [Green Version]
- Bittencourt, S.; Dubiela, F.P.; Queiroz, C.; Covolan, L.; Andrade, D.; Lozano, A.; Mello, L.E.; Hamani, C. Microinjection of GABAergic agents into the anterior nucleus of the thalamus modulates pilocarpine-induced seizures and status epilepticus. Seizure 2010, 19, 242–246. [Google Scholar] [CrossRef] [Green Version]
- Mirski, M.A.; Ferrendelli, J.A. Anterior thalamic mediation of generalized pentylenetetrazol seizures. Brain Res. 1986, 399, 212–223. [Google Scholar] [CrossRef]
- Cassidy, R.M.; Gale, K. Mediodorsal thalamus plays a critical role in the development of limbic motor seizures. J. Neurosci. 1998, 18, 9002–9009. [Google Scholar] [CrossRef]
- Bertram, E.H. Extratemporal lobe circuits in temporal lobe epilepsy. Epilepsy Behav. 2014, 38, 13–18. [Google Scholar] [CrossRef]
- Bertram, E.H.; Zhang, D.; Williamson, J.M. Multiple roles of midline dorsal thalamic nuclei in induction and spread of limbic seizures. Epilepsia 2008, 49, 256–268. [Google Scholar] [CrossRef]
- Patel, S.; Millan, M.H.; Meldrum, B.S. Decrease in excitatory transmission within the lateral habenula and the mediodorsal thalamus protects against limbic seizures in rats. Exp. Neurol. 1988, 101, 63–74. [Google Scholar] [CrossRef]
- Garant, D.S.; Xu, S.G.; Sperber, E.F.; Moshe, S.L. The influence of thalamic GABA transmission on the susceptibility of adult rats to flurothyl induced seizures. Epilepsy Res. 1993, 15, 185–192. [Google Scholar] [CrossRef]
- Shehab, S.; Guadagno, J.; Ferguson, K.; Redgrave, P. Regional distribution of the anticonvulsant and behavioural effects of bicuculline injected into the pontine reticular formation of rats. Eur. J. Neurosci. 1997, 9, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Shehab, S.; Alzigali, L.; Madathil, M.; Redgrave, P. Pharmacological evidence for an anticonvulsant relay in the rat ventromedial medulla. Eur. J. Neurosci. 2007, 26, 2585–2594. [Google Scholar] [CrossRef] [PubMed]
- Wicker, E.; Beck, V.C.; Kulick-Soper, C.; Kulick-Soper, C.V.; Hyder, S.K.; Campos-Rodriguez, C.; Khan, T.; N’Gouemo, P.; Forcelli, P.A. Descending projections from the substantia nigra pars reticulata differentially control seizures. Proc. Natl. Acad. Sci. USA 2019, 116, 27084–27094. [Google Scholar] [CrossRef] [PubMed]
- De Sarro, G.; Meldrum, B.S.; De Sarro, A.; Patel, S. Excitatory neurotransmitters in the lateral habenula and pedunculopontine nucleus of rat modulate limbic seizures induced by pilocarpine. Brain Res. 1992, 591, 209–222. [Google Scholar] [CrossRef]
- Hamani, C.; Sakabe, S.; Bortolotto, Z.A.; Cavalheiro, E.A.; Mello, L.E. Inhibitory role of the zona incerta in the pilocarpine model of epilepsy. Epilepsy Res. 2002, 49, 73–80. [Google Scholar] [CrossRef]
- Remy, S.; Beck, H. Molecular and cellular mechanisms of pharmacoresistance in epilepsy. Brain 2006, 129, 18–35. [Google Scholar] [CrossRef] [Green Version]
- Fang, M.; Xi, Z.Q.; Wu, Y.; Wang, X.F. A new hypothesis of drug refractory epilepsy: Neural network hypothesis. Med. Hypotheses 2011, 76, 871–876. [Google Scholar] [CrossRef]
- Ghosh, C.; Puvenna, V.; Gonzalez-Martinez, J.; Janigro, D.; Marchi, N. Blood-brain barrier P450 enzymes and multidrug transporters in drug resistance: A synergistic role in neurological diseases. Curr. Drug Metab. 2011, 12, 742–749. [Google Scholar] [CrossRef] [Green Version]
- Gambardella, A.; Labate, A.; Mumoli, L.; Lopes-Cendes, I.; Cendes, F. Role of Pharmacogenomics in Antiepileptic Drug Therapy: Current Status and Future Perspectives. Curr. Pharm. Des. 2017, 23, 5760–5765. [Google Scholar] [CrossRef] [PubMed]
- Balestrini, S.; Sisodiya, S.M. Pharmacogenomics in epilepsy. Neurosci. Lett. 2018, 667, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Schmidt, D. Experimental and clinical evidence for loss of effect (tolerance) during prolonged treatment with antiepileptic drugs. Epilepsia 2006, 47, 1253–1284. [Google Scholar] [CrossRef] [PubMed]
- Hardman, C.D.; Henderson, J.M.; Finkelstein, D.I.; Horne, M.K.; Paxinos, G.; Halliday, G.M. Comparison of the basal ganglia in rats, marmosets, macaques, baboons, and humans: Volume and neuronal number for the output, internal relay, and striatal modulating nuclei. J. Comp. Neurol. 2002, 445, 238–255. [Google Scholar] [CrossRef]
- Iadarola, M.J.; Gale, K. Cellular compartments of GABA in brain and their relationship to anticonvulsant activity. Mol. Cell Biochem. 1981, 39, 305–329. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gernert, M.; Feja, M. Bypassing the Blood–Brain Barrier: Direct Intracranial Drug Delivery in Epilepsies. Pharmaceutics 2020, 12, 1134. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12121134
Gernert M, Feja M. Bypassing the Blood–Brain Barrier: Direct Intracranial Drug Delivery in Epilepsies. Pharmaceutics. 2020; 12(12):1134. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12121134
Chicago/Turabian StyleGernert, Manuela, and Malte Feja. 2020. "Bypassing the Blood–Brain Barrier: Direct Intracranial Drug Delivery in Epilepsies" Pharmaceutics 12, no. 12: 1134. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12121134