The Development of an In Vitro Horizontal Diffusion Cell to Monitor Nasal Powder Penetration Inline
Abstract
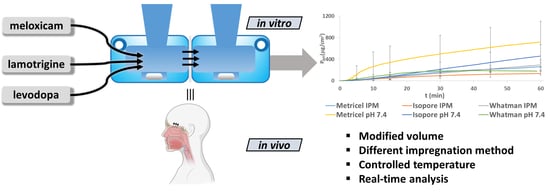
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Particle Size Determination of the APIs
2.3. Hydrophilicity Determination of the Membranes with Contact Angle Measurements
2.4. Factors Influencing the Results of the Diffusion Measurements
2.5. In Vitro Diffusion Measurement by the Horizontal Cells
2.6. Offline Spectrophotometric Measurements
2.7. Inline Measurements with a Probe Connected to a Spectrophotometer with Optical Fiber
3. Results and Discussion
3.1. Evaluation of the Factors Affecting the Adaptability of the Horizontal Diffusion Cells for the Penetration Measurements of Nasal Powders
3.2. Measurement of the Size Distribution of Applied Model APIs
3.3. Determination of Surface Free Energy and Polarity of Membranes Using Wu’s Method
3.4. The Design of the Horizontal Diffusion Cell Appropriate for the Investigation of Nasal Powders
3.5. Determination of Calibration Curves by Offline and Inline Monitoring
3.6. In Vitro Diffusion Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pires, A.; Fortuna, A.; Alves, G.; Falcão, A. Intranasal Drug Delivery: How, Why and What for? J. Pharm. Pharm. Sci. 2009, 12, 288. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-F.; Fawcett, J.R.; Hanson, L.R.; Frey, W.H. The window of opportunity for treatment of focal cerebral ischemic damage with noninvasive intranasal insulin-like growth factor-I in rats. J. Stroke Cerebrovasc. Dis. 2004, 13, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Schmidt, M.C.; Peter, H.; Lang, S.R.; Ditzinger, G.; Merkle, H.P. In vitro cell models to study nasal mucosal permeability and metabolism. Adv. Drug Deliv. Rev. 1998, 29, 51–79. [Google Scholar] [CrossRef]
- Gieszinger, P.; Csóka, I.; Pallagi, E.; Katona, G.; Jójárt-Laczkovich, O.; Szabó-Révész, P.; Ambrus, R. Preliminary study of nanonized lamotrigine containing products for nasal powder formulation. DDDT 2017, 11, 2453–2466. [Google Scholar] [CrossRef] [Green Version]
- Alexander, A.; Saraf, S. Nose-to-brain drug delivery approach: A key to easily accessing the brain for the treatment of Alzheimer’s disease. Neural. Regen. Res. 2018, 13, 2102. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; de Melchiades, G.L.; Figueiró, F.; Battastini, A.M.O.; Teixeira, H.F.; Koester, L.S. Validation of an HPLC-UV method for analysis of Kaempferol-loaded nanoemulsion and its application to in vitro and in vivo tests. J. Pharm. Biomed. Anal. 2017, 145, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Vasa, D.M.; O’Donnell, L.A.; Wildfong, P.L.D. Influence of Dosage Form, Formulation, and Delivery Device on Olfactory Deposition and Clearance: Enhancement of Nose-to-CNS Uptake. J. Pharm. Innov. 2015, 10, 200–210. [Google Scholar] [CrossRef]
- Illum, L. Nasal drug delivery—possibilities, problems and solutions. J. Control. Release 2003, 87, 187–198. [Google Scholar] [CrossRef]
- Scherließ, R.; Mönckedieck, M.; Young, K.; Trows, S.; Buske, S.; Hook, S. First in vivo evaluation of particulate nasal dry powder vaccine formulations containing ovalbumin in mice. Int. J. Pharm. 2015, 479, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Tepper, S.J.; Johnstone, M.R. Breath-powered sumatriptan dry nasal powder: An intranasal medication delivery system for acute treatment of migraine. MDER 2018, 11, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, A.; Furubayashi, T.; Yamasaki, H.; Takano, K.; Kawakami, M.; Kimura, S.; Inoue, D.; Katsumi, H.; Sakane, T.; Yamamoto, A. The Enhancement of Nasal Drug Absorption from Powder Formulations by the Addition of Sodium Carboxymethyl Cellulose. IEEE Trans. Nanobiosci. 2016, 15, 798–803. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, H.; Chen, Y.; Gibson, C.; Heimbach, T.; Parrott, N.; Peters, S.; Snoeys, J.; Upreti, V.; Zheng, M.; Hall, S. Physiologically based pharmacokinetic modeling in drug discovery and development: A pharmaceutical industry perspective. Clin. Pharm. 2015, 97, 247–262. [Google Scholar] [CrossRef]
- Booth, R.; Kim, H. Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB). Lab. Chip 2012, 12, 1784. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Busato, L.; Pozzoli, M.; Ghadiri, M.; Ong, H.X.; Young, P.M.; Manfredini, S.; Traini, D. In vitro characterization of physico-chemical properties, cytotoxicity, bioactivity of urea-crosslinked hyaluronic acid and sodium ascorbyl phosphate nasal powder formulation. Int. J. Pharm. 2019, 558, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Borbás, E.; Balogh, A.; Bocz, K.; Müller, J.; Kiserdei, É.; Vigh, T.; Sinkó, B.; Marosi, A.; Halász, A.; Dohányos, Z.; et al. In vitro dissolution–permeation evaluation of an electrospun cyclodextrin-based formulation of aripiprazole using μFluxTM. Int. J. Pharm. 2015, 491, 180–189. [Google Scholar] [CrossRef]
- Bakand, S.; Winder, C.; Khalil, C.; Hayes, A. An experimental in vitro model for dynamic direct exposure of human cells to airborne contaminants. Toxicol. Lett. 2006, 165, 1–10. [Google Scholar] [CrossRef]
- Bakand, S.; Winder, C.; Hayes, A. Comparative in vitro cytotoxicity assessment of selected gaseous compounds in human alveolar epithelial cells. Toxicol. Vitr. 2007, 21, 1341–1347. [Google Scholar] [CrossRef]
- Córdoba-Dı́az, M.; Nova, M.; Elorza, B.; Córdoba-Dı́az, D.; Chantres, J.R.; Córdoba-Borrego, M. Validation protocol of an automated in-line flow-through diffusion equipment for in vitro permeation studies. J. Control. Release 2000, 69, 357–367. [Google Scholar] [CrossRef]
- Xiang, J.; Fang, X.; Li, X. Transbuccal delivery of 2′,3′-dideoxycytidine: In vitro permeation study and histological investigation. Int. J. Pharm. 2002, 231, 57–66. [Google Scholar] [CrossRef]
- Pund, S.; Rasve, G.; Borade, G. Ex vivo permeation characteristics of venlafaxine through sheep nasal mucosa. Eur. J. Pharm. Sci. 2013, 48, 195–201. [Google Scholar] [CrossRef]
- Trbojevich, R.A.; Fernandez, A.; Watanabe, F.; Mustafa, T.; Bryant, M.S. Comparative study of silver nanoparticle permeation using Side-Bi-Side and Franz diffusion cells. J. Nanopart. Res. 2016, 18, 55. [Google Scholar] [CrossRef]
- Nardviriyakul, N.; Wurster, D.E.; Donovan, M.D. Determination of Diffusion Coefficients of Sodium p-aminosalicylate in Sheep Nasal Mucosae and Dialysis Membranes by Fourier Transform Infrared Horizontal Attenuated Total Reflectance Spectroscopy. J. Pharm. Sci. 1997, 86, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kiss, T.; Alapi, T.; Varga, G.; Bartos, C.; Ambrus, R.; Szabó-Révész, P.; Katona, G. Interaction Studies Between Levodopa and Different Excipients to Develop Coground Binary Mixtures for Intranasal Application. J. Pharm. Sci. 2019, 108, 2552–2560. [Google Scholar] [CrossRef] [PubMed]
- Jug, M.; Hafner, A.; Lovrić, J.; Kregar, M.L.; Pepić, I.; Vanić, Ž.; Cetina-Čižmek, B.; Filipović-Grčić, J. An overview of in vitro dissolution/release methods for novel mucosal drug delivery systems. J. Pharm. Biomed. Anal. 2018, 147, 350–366. [Google Scholar] [CrossRef] [PubMed]
- Clementino, A.; Sonvico, F. Development and validation of a RP-HPLC method for the simultaneous detection and quantification of simvastatin’s isoforms and coenzyme Q10 in lecithin/chitosan nanoparticles. J. Pharm. Biomed. Anal. 2018, 155, 33–41. [Google Scholar] [CrossRef]
- Tiozzo Fasiolo, L.; Manniello, M.D.; Tratta, E.; Buttini, F.; Rossi, A.; Sonvico, F.; Bortolotti, F.; Russo, P.; Colombo, G. Opportunity and challenges of nasal powders: Drug formulation and delivery. Eur. J. Pharm. Sci. 2018, 113, 2–17. [Google Scholar] [CrossRef]
- Gizurarson, S. Anatomical and Histological Factors Affecting Intranasal Drug and Vaccine Delivery. CDD 2012, 9, 566–582. [Google Scholar] [CrossRef] [Green Version]
- Gizurarson, S. The relevance of nasal physiology to the design of drug absorption studies. Adv. Drug Deliv. Rev. 1993, 11, 329–347. [Google Scholar] [CrossRef]
- Horváth, T.; Ambrus, R.; Szabó-Révész, P. Investigation of permeability of intranasal formulations using Side-Bi-Side horizontal diffusion cell. Acta Pharm. Hung. 2015, 85, 19–28. [Google Scholar]
- Horváth, T.; Ambrus, R.; Völgyi, G.; Budai-Szűcs, M.; Márki, Á.; Sipos, P.; Bartos, C.; Seres, A.; Sztojkov-Ivanov, A.; Takács-Novák, K.; et al. Effect of solubility enhancement on nasal absorption of meloxicam. Eur. J. Pharm. Sci. 2016, 95, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Name | Application | Direction of Diffusion | Volume and Measurement Conditions |
|---|---|---|---|
| μFLUX™ diffusion cell—in situ UV monitoring optical system (Pion Inc., Billerica, Massachusetts, United States) [17] | Diffusion measurement with artificial membrane | Horizontal | Both phases: 10–13 mL Real-time analysis is possible |
| Navicyte Vertical Diffusion Chamber System (Harvard Apparatus, Holliston, Massachusetts, United States) | Cells, ex vivo tissue investigation Intestinal and nasal permeability | Vertical | Both phases: variable Real-time analysis is possible |
| Navicyte Horizontal Diffusion Chamber System (Harvard Apparatus, Holliston, Massachusetts, United States) [18,19] | Tissues contacting with air (nasal, pulmonary, dermal) permeability | Horizontal | Both phases: variable Real-time measurement is possible |
| In-Line Cell (PermeGear Inc., Hellertown, Pennsylvania, United States) [20,21] | Permeability of transdermal and buccal formulations | Vertical | Acceptor phase: variable Donor phase: 100 mL Real-time analysis is possible |
| Franz vertical diffusion cell (Hanson Research, Chatsworth, California, USA) [16,22] | Permeability of transdermal formulations | Vertical | Donor phase: 300 µL Acceptor phase: 7 mL No real-time analysis |
| Side-Bi-Side™ horizontal diffusion cell (PermeGear Inc., Hellertown, Pennsylvania, United States) [23,24] | Blood–brain barrier investigations Permeability of nasal formulations | Horizontal | Both phases: 3 mL Real-time analysis is possible |
| API | d (0.1) | d(0.5) | d(0.9) |
|---|---|---|---|
| MEL | 2.99 ± 0.17 | 10.76 ± 0.66 | 31.72 ± 2.93 |
| LAM | 2.82 ± 0.05 | 11.71 ± 0.41 | 63.37 ± 7.69 |
| LEV | 4.84 ± 0.02 | 22.25 ± 0.24 | 81.53 ± 3.28 |
| Membrane | θ water (°) | θ diiodomethane (°) | γ (mN/m) | γp (mN/m) | γd (mN/m) | Polarity (%) |
|---|---|---|---|---|---|---|
| Metricel® | 29.3 | 3.4 | 76.23 | 30.41 | 45.82 | 39.89 |
| Isopore™ | 40 | 8.1 | 71.3 | 25.8 | 45.49 | 36.19 |
| Whatman™ | 16.2 | 14.5 | 79.6 | 35.24 | 44.36 | 44.27 |
| Change in Design | The Design of Side-Bi-Side™ Horizontal Diffusion Cell | The Design of New Horizontal Cell |
|---|---|---|
| Cell volume | 3 mL | 9 mL |
| Magnetic stirring bars | In the sample space | In the hollow under the sample space |
| Sampling ports | Too narrow sampling port for probe input | The design of sampling port for probe input |
| API | Offline Measurements | Inline Measurements | ||||
|---|---|---|---|---|---|---|
| Calibration Equation | LOD/LOQ (μg/mL) | ULOQ (μg/mL) | Calibration Equation | LOD/ LOQ (μg/mL) | ULOQ (μg/mL) | |
| MEL | y = 0.04849x | 0.05296/0.1605 | 39.09 | y = 0.04658x | 0.08726/0.2644 | 40.00 |
| LAM | y = 0.02745x | 0.09062/0.2746 | 20.03 | y = 0.02708x | 0.05602/0.1698 | 20.62 |
| LEV | y = 0.01350x | 1.736/5.262 | 90.41 | y = 0.01349x | 0.2265/0.6863 | 100.04 |
| API | Connectivity | Offline | Inline | ||||
|---|---|---|---|---|---|---|---|
| Impregnation Agent/Membrane | Metricel® | Isopore™ | Whatman™ | Metricel® | Isopore™ | Whatman™ | |
| MEL | pH = 7.4 | 0.91 | 2.20 | 1.18 | −0.37 | 2.44 | 3.12 |
| IPM | 1.25 | 8.54 | 5.51 | 69.41 | 32.00 | 39.62 | |
| LAM | pH = 7.4 | 55.21 | 41.73 | 30.17 | 0.18 | 53.48 | 45.20 |
| IPM | 2.38 | 15.84 | 25.56 | 8.98 | 11.12 | 57.04 | |
| LEV | pH = 7.4 | 506.16 | 476.67 | 336.98 | 341.86 | 81.81 | 115.60 |
| IPM | 20.70 | 13.44 | 165.59 | 66.20 | 50.21 | 63.19 | |
| API | Connectivity | Offline | Inline | ||||
|---|---|---|---|---|---|---|---|
| Impregnation Agent/Membrane | Metricel® | Isopore™ | Whatman™ | Metricel® | Isopore™ | Whatman™ | |
| MEL | pH = 7.4 | 81.44 | 40.53 | 93.00 | 84.53 | 21.27 | 62.97 |
| IPM | 66.33 | 57.79 | 78.98 | 14.72 | 22.86 | 38.77 | |
| LAM | pH = 7.4 | 17.00 | 63.02 | 22.09 | 89.23 | 76.39 | 35.92 |
| IPM | 58.66 | 14.15 | 54.90 | 16.67 | 27.67 | 19.54 | |
| LEV | pH = 7.4 | 24.38 | 12.11 | 53.73 | 83.14 | 66.42 | 26.84 |
| IPM | 8.57 | 66.41 | 31.2 | 12.36 | 65.54 | 51.21 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gieszinger, P.; Kiss, T.; Szabó-Révész, P.; Ambrus, R. The Development of an In Vitro Horizontal Diffusion Cell to Monitor Nasal Powder Penetration Inline. Pharmaceutics 2021, 13, 809. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13060809
Gieszinger P, Kiss T, Szabó-Révész P, Ambrus R. The Development of an In Vitro Horizontal Diffusion Cell to Monitor Nasal Powder Penetration Inline. Pharmaceutics. 2021; 13(6):809. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13060809
Chicago/Turabian StyleGieszinger, Péter, Tamás Kiss, Piroska Szabó-Révész, and Rita Ambrus. 2021. "The Development of an In Vitro Horizontal Diffusion Cell to Monitor Nasal Powder Penetration Inline" Pharmaceutics 13, no. 6: 809. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13060809