Development of Jelly Loaded with Nanogel Containing Natural L-Dopa from Mucuna pruriens Seed Extract for Neuroprotection in Parkinson’s Disease
Abstract
:1. Introduction
2. Materials
3. Methods
3.1. M. pruriens Seed Identification, Extraction and Spray Drying
3.2. Phytochemical Screening of M. pruriens Seed Extract
3.2.1. Flavonoids Test
3.2.2. Tannins and Phenolics Test
3.2.3. Alkaloids Test
3.2.4. Terpenoids Test
3.2.5. Saponins Test
3.3. Quantitative Analysis of Phenolic Compounds and Total Flavonoid Content in M. pruriens Seed Extract
3.4. Chemical Analysis of L-Dopa in M. pruriens Seed Extract
3.4.1. Thin Layer Chromatography
3.4.2. High Performance Liquid Chromatography
3.5. Determination of M. pruriens Seed Extract Antioxidant Activity
3.5.1. DPPH Free-Radical Scavenging Activity
3.5.2. ABTS Free-Radical Scavenging Activity
3.5.3. Ferric Reducing Antioxidant Power
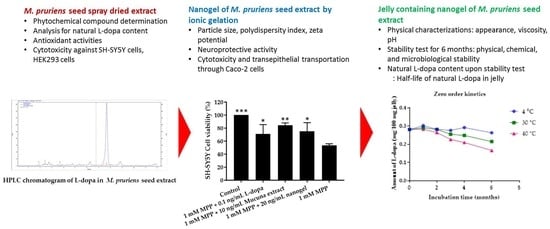
3.6. Preparation and Characterization of M. pruriens Seed-Extract Nanogel
3.7. Neuroprotective Assay of M. pruriens Seed Extract and Nanogel against SH-SY5Y Cells
3.8. Cytotoxicity Assay of M. pruriens Seed Extract and Nanogel against HEK293 Cells
3.9. Permeability Study of M. pruriens Seed-Extract Nanogel across Caco-2 Cells
3.10. Development of Jelly Containing M. pruriens Seed-Extract Nanogel
3.10.1. Formulation of Jelly Containing M. pruriens Seed-Extract Nanogel
3.10.2. Physical and Chemical Stabilities of Jelly Containing M. pruriens Seed-Extract Nanogel
3.10.3. Microbiological Stability of Jelly Containing M. pruriens Seed-Extract Nanogel
3.11. Statistical Analysis
4. Results and Discussion
4.1. Yield of M. pruriens Seed Extraction
4.2. Phytochemical Compounds in Spray-Dried M. pruriens Seed Extract
4.3. Total Phenolic and Total Flavonoid Contents in Spray-Dried M. pruriens Seed Extract
4.4. Qualitative and Quantitative Analysis of L-Dopa in M. pruriens Seed Extract
4.4.1. TLC Analysis of L-Dopa in M. pruriens Seed Extract
4.4.2. HPLC Analysis of L-Dopa in M. pruriens Seed Extract
4.5. Antioxidant Activity of M. pruriens Seed Extract
4.6. Particle Size, Size Distribution, Zeta Potential, and Drug Release of M. pruriens Seed-Extract Nanogel
4.7. Neuroprotective Effect of M. pruriens Seed Extract and Nanogel on SH-SY5Y Cells
4.8. Cytotoxic Effect of M. pruriens Seed Extract and Nanogel on Renal Cells
4.9. Permeability of M. pruriens Seed-Extract Nanogel through Caco-2 Cell Monolayer
4.10. Characterization and Stability of M. pruriens Seed Extract and Jelly Containing M. pruriens Seed Extract Nanogel
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pathak-Gandhi, N.; Vaidya, A.D. Management of Parkinson’s disease in Ayurveda: Medicinal plants and adjuvant measures. J. Ethnopharmacol. 2017, 197, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Kamkaen, N.; Chittasupho, C.; Vorarat, S.; Tadtong, S.; Phrompittayarat, W.; Okonogi, S.; Kwankhao, P. Mucuna pruriens Seed Aqueous Extract Improved Neuroprotective and Acetylcholinesterase Inhibitory Effects Compared with Synthetic L-Dopa. Molecules 2022, 27, 3131. [Google Scholar] [CrossRef]
- Poddighe, S.; De Rose, F.; Marotta, R.; Ruffilli, R.; Fanti, M.; Secci, P.P.; Mostallino, M.C.; Setzu, M.D.; Zuncheddu, M.A.; Collu, I.; et al. Mucuna pruriens (Velvet bean) Rescues Motor, Olfactory, Mitochondrial and Synaptic Impairment in PINK1B9 Drosophila melanogaster Genetic Model of Parkinson’s Disease. PLoS ONE 2014, 9, e110802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katzenschlager, R.; Evans, A.; Manson, A.; Patsalos, P.N.; Ratnaraj, N.; Watt, H.; Timmermann, L.; Van der Giessen, R.; Lees, A.J. Mucuna pruriens in Parkinson’s disease: A double blind clinical and pharmacological study. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1672–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahajani, S.S.; Doshi, V.J.; Parikh, K.M.; Manyam, B.V. Bioavailability of L-DOPA from HP-200—A Formulation of Seed Powder of Mucuna pruriens (Bak): A Pharmacokinetic and Pharmacodynamic Study. Phytother. Res. 1996, 10, 254–256. [Google Scholar] [CrossRef]
- Khan, R.A.; Arif, M.; Sherwani, B.; Ahmed, M.M. (Eds.) Acute and Sub Chronic Toxicity of Mucuna pruriens, Cinnamomum zeylanicum, Myristica fragrans and their Effects on Hematological Parameters. Aust. J. Basic Appl. Sci. 2013, 7, 641–647. [Google Scholar]
- Hussian, G.; Manyam, B.V. Mucuna pruriens proves more effective than L-DOPA in Parkinson’s disease animal model. Phytother. Res. 1997, 11, 419–423. [Google Scholar] [CrossRef]
- Lieu, C.A.; Venkiteswaran, K.; Gilmour, T.P.; Rao, A.N.; Petticoffer, A.C.; Gilbert, E.V.; Deogaonkar, M.; Manyam, B.V.; Subramanian, T. The Antiparkinsonian and Antidyskinetic Mechanisms of Mucuna pruriens in the MPTP-Treated Nonhuman Primate. Evid. Based Complement. Alternat. Med. 2012, 2012, 840247. [Google Scholar] [CrossRef] [Green Version]
- Lieu, C.A.; Kunselman, A.R.; Manyam, B.V.; Venkiteswaran, K.; Subramanian, T. A water extract of Mucuna pruriens provides long-term amelioration of parkinsonism with reduced risk for dyskinesias. Parkinsonism Relat. Disord. 2010, 16, 458–465. [Google Scholar] [CrossRef] [Green Version]
- Liebert, M.A. An alternative medicine treatment for Parkinson’s disease: Results of a multicenter clinical trial. HP-200 in Parkinson’s Disease Study Group. J. Altern. Complement. Med. 1995, 1, 249–255. [Google Scholar]
- Goldenberg, M.M. Overview of drugs used for epilepsy and seizures: Etiology, diagnosis, and treatment. Pharm. Ther. 2010, 35, 392–415. [Google Scholar]
- Chittasupho, C.; Kamkaen, N. Development and Characterization of Oral Mucuna pruriens Seed Extract Jelly. Key Eng. Mater. 2021, 901, 61–66. [Google Scholar] [CrossRef]
- Chiangnoon, R.; Samee, W.; Uttayarat, P.; Jittachai, W.; Ruksiriwanich, W.; Sommano, S.R.; Athikomkulchai, S.; Chittasupho, C. Phytochemical Analysis, Antioxidant, and Wound Healing Activity of Pluchea indica L. (Less) Branch Extract Nanoparticles. Molecules 2022, 27, 638. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, E.; Salim, K.A.; Lim, L.B.L. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J. King Saud Univ. Sci. 2015, 27, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Tunit, P.; Thammarat, P.; Okonogi, S.; Chittasupho, C. Hydrogel Containing Borassus flabellifer L. Male Flower Extract for Antioxidant, Antimicrobial, and Anti-Inflammatory Activity. Gels 2022, 8, 126. [Google Scholar] [CrossRef]
- Modi, K.P.; Patel, N.M.; Goyal, R.K. Estimation of L-dopa from Mucuna pruriens Linn and formulations containing M. pruriens by HPTLC method. Chem. Pharm. Bull. 2008, 56, 357–359. [Google Scholar] [CrossRef] [Green Version]
- Chittasupho, C.; Tadtong, S.; Vorarat, S.; Kamkaen, N. Physical, Chemical, and Microbiological Stability of Mucuna pruriens Effervescent Powders and Suspension. Key Eng. Mater. 2020, 859, 145–150. [Google Scholar] [CrossRef]
- Wongsukkasem, N.; Soynark, O.; Suthakitmanus, M.; Chongdiloet, E.; Chairattanapituk, C.; Vattanikitsiri, P.; Hongratanaworakit, T.; Tadtong, S. Antiacne-causing Bacteria, Antioxidant, Anti-Tyrosinase, Anti-Elastase and Anti-Collagenase Activities of Blend Essential Oil comprising Rose, Bergamot and Patchouli Oils. Nat. Prod. Commun. 2018, 13, 1934578X1801300529. [Google Scholar] [CrossRef] [Green Version]
- Sang, Q.; Liu, X.; Wang, L.; Qi, L.; Sun, W.; Wang, W.; Sun, Y.; Zhang, H. Curcumin Protects an SH-SY5Y Cell Model of Parkinson’s Disease against Toxic Injury by Regulating HSP90. Cell Physiol. Biochem. 2018, 51, 681–691. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, Y.; Jin, L.W.; Ye, H.Y.; Liu, G. Cytotoxicity and Genotoxicity in Human Embryonic Kidney Cells Exposed to Surface Modify Chitosan Nanoparticles Loaded with Curcumin. AAPS Pharm. Sci. Tech. 2016, 17, 1347–1352. [Google Scholar] [CrossRef] [Green Version]
- Chittasupho, C.; Junmahasathien, T.; Chalermmongkol, J.; Wongjirasakul, R.; Leesawat, P.; Okonogi, S. Suppression of Intracellular Reactive Oxygen Species in Human Corneal Epithelial Cells via the Combination of Quercetin Nanoparticles and Epigallocatechin Gallate and In Situ Thermosensitive Gel Formulation for Ocular Drug Delivery. Pharmaceuticals 2021, 14, 679. [Google Scholar] [CrossRef] [PubMed]
- Chittasupho, C.; Thongnopkoon, T.; Burapapisut, S.; Charoensukkho, C.; Shuwisitkul, D.; Samee, W. Stability, permeation, and cytotoxicity reduction of capsicum extract nanoparticles loaded hydrogel containing wax gourd extract. Saudi Pharm. J. 2020, 28, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Buranasukhon, W.; Athikomkulchai, S.; Tadtong, S.; Chittasupho, C. Wound healing activity of Pluchea indica leaf extract in oral mucosal cell line and oral spray formulation containing nanoparticles of the extract. Pharm. Biol. 2017, 55, 1767–1774. [Google Scholar] [CrossRef] [Green Version]
- Sathiyanarayanan, L.; Arulmozhi, S. Mucuna pruriens Linn.—A comprehensive review. Pharmacogn. Rev. 2007, 1, 157. [Google Scholar]
- Lampariello, L.R.; Cortelazzo, A.; Guerranti, R.; Sticozzi, C.; Valacchi, G. The Magic Velvet Bean of Mucuna pruriens. J. Tradit Complement. Med. 2012, 2, 331–339. [Google Scholar] [CrossRef]
- Pathania, R.; Chawla, P.; Khan, H.; Kaushik, R.; Khan, M.A. An assessment of potential nutritive and medicinal properties of Mucuna pruriens: A natural food legume. 3 Biotech 2020, 10, 261. [Google Scholar] [CrossRef]
- Misra, L.; Wagner, H. Alkaloidal constituents of Mucuna pruriens seeds. Phytochemistry 2004, 65, 2565–2567. [Google Scholar] [CrossRef]
- Shanmugavel, G.; Krishnamoorthy, G. (Eds.) Nutraceutical and Phytochemical Investigation of Mucuna pruriens Seed. Pharma Innov. 2018, 7, 273–278. [Google Scholar]
- Longhi, J.G.; Pérez, E.S.; Lima, J.J.; Cândido, L.M.B. In vitro evaluation of Mucuna pruriens (L.) DC. antioxidant activity. Braz. J. Pharm. Sci. 2011, 47, 535–544. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Vijayakumari, K.; Janardhanan, K. Chemical Composition and Protein Quality of the Little-Known Legume, Velvet Bean (Mucuna pruriens (L.) DC.). J. Agric. Food Chem. 1996, 44, 2636–2641. [Google Scholar] [CrossRef]
- Jimoh, M.A.; Idris, O.A.; Jimoh, M.O. Cytotoxicity, Phytochemical, Antiparasitic Screening, and Antioxidant Activities of Mucuna pruriens (Fabaceae). Plants 2020, 9, 1249. [Google Scholar] [CrossRef]
- Theansungnoen, T.; Nitthikan, N.; Wilai, M.; Chaiwut, P.; Kiattisin, K.; Intharuksa, A. Phytochemical Analysis and Antioxidant, Antimicrobial, and Antiaging Activities of Ethanolic Seed Extracts of Four Mucuna Species. Cosmetics 2022, 9, 14. [Google Scholar] [CrossRef]
- Dhanasekaran, M.; Tharakan, B.; Manyam, B.V. Antiparkinson drug—Mucuna pruriens shows antioxidant and metal chelating activity. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2008, 22, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Adefegha, S.A.; Oboh, G.; Oyeleye, S.I.; Dada, F.A.; Ejakpovi, I.; Boligon, A.A. Cognitive Enhancing and Antioxidative Potentials of Velvet Beans (Mucuna pruriens) and Horseradish (Moringa oleifera) Seeds Extracts: A Comparative Study. J. Food Biochem. 2017, 41, e12292. [Google Scholar] [CrossRef]
- Wang, H.; Deng, H.; Gao, M.; Zhang, W. Self-Assembled Nanogels Based on Ionic Gelation of Natural Polysaccharides for Drug Delivery. Front. Bioeng. Biotechnol. 2021, 9, 703559. [Google Scholar] [CrossRef]
- Al-Ahmad, A.J.; Patel, R.; Palecek, S.P.; Shusta, E.V. Hyaluronan impairs the barrier integrity of brain microvascular endothelial cells through a CD44-dependent pathway. J. Cereb. Blood Flow Metab. 2019, 39, 1759–1775. [Google Scholar] [CrossRef]
- Yuan, J.; Gao, Y.; Wang, X.; Liu, H.; Che, X.; Xu, L.; Yang, Y.; Wang, Q.; Wang, Y.; Li, S. The load and release characteristics on a strong cationic ion-exchange fiber: Kinetics, thermodynamics, and influences. Drug Des. Dev. Ther. 2014, 8, 945–955. [Google Scholar]
- Sahu, P.; Kashaw, S.K.; Sau, S.; Kushwah, V.; Jain, S.; Agrawal, R.K.; Iyer, A.K. pH triggered and charge attracted nanogel for simultaneous evaluation of penetration and toxicity against skin cancer: In-vitro and ex-vivo study. Int. J. Biol. Macromol. 2019, 128, 740–751. [Google Scholar] [CrossRef]
- Xu, X.; Jha, A.K.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Hyaluronic Acid-Based Hydrogels: From a Natural Polysaccharide to Complex Networks. Soft Matter 2012, 8, 3280–3294. [Google Scholar] [CrossRef] [Green Version]
- Rai, S.N.; Birla, H.; Singh, S.S.; Zahra, W.; Patil, R.R.; Jadhav, J.P.; Gedda, M.R.; Singh, S.P. Mucuna pruriens Protects against MPTP Intoxicated Neuroinflammation in Parkinson’s Disease through NF-κB/pAKT Signaling Pathways. Front. Aging Neurosci. 2017, 9, 421. [Google Scholar] [CrossRef] [Green Version]
- Stockdale, T.P.; Challinor, V.L.; Lehmann, R.P.; De Voss, J.J.; Blanchfield, J.T. Caco-2 Monolayer Permeability and Stability of Chamaelirium luteum (False Unicorn) Open-Chain Steroidal Saponins. ACS Omega 2019, 4, 7658–7666. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Lennernas, H.; Welage, L.S.; Barnett, J.L.; Landowski, C.P.; Foster, D.; Lee, K.D.; Amidon, G.L. Comparison of human duodenum and Caco-2 gene expression profiles for 12,000 gene sequences tags and correlation with permeability of 26 drugs. Pharm. Res. 2002, 19, 1400–1416. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Borchardt, R.T. Mechanism of L-alpha-methyldopa transport through a monolayer of polarized human intestinal epithelial cells (Caco-2). Pharm. Res. 1990, 7, 1313–1319. [Google Scholar] [CrossRef]
- Vieira-Coelho, M.A.; Soares-Da-Silva, P. Uptake and intracellular fate of l-DOPA in a human intestinal epithelial cell line: Caco-2. Am. J. Physiol.-Cell Physiol. 1998, 275, C104–C112. [Google Scholar] [CrossRef] [PubMed]
- Pappert, E.J.; Buhrfiend, C.; Lipton, J.W.; Carvey, P.M.; Stebbins, G.T.; Goetz, C.G. Levodopa stability in solution: Time course, environmental effects, and practical recommendations for clinical use. Mov. Disord. 1996, 11, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Isariebel, Q.-P.; Carine, J.-L.; Ulises-Javier, J.-H.; Anne-Marie, W.; Henri, D. Sonolysis of levodopa and paracetamol in aqueous solutions. Ultrason. Sonochem. 2009, 16, 610–616. [Google Scholar] [CrossRef] [Green Version]
- Dai, Q.; Xia, Y.; Sun, C.; Weng, M.; Chen, J.; Wang, J.; Chen, J. Electrochemical degradation of levodopa with modified PbO2 electrode: Parameter optimization and degradation mechanism. Chem. Eng. J. 2014, 245, 359–366. [Google Scholar] [CrossRef]
- Pulikkalpura, H.; Kurup, R.; Mathew, P.J.; Baby, S. Levodopa in Mucuna pruriens and its degradation. Sci. Rep. 2015, 5, 11078. [Google Scholar] [CrossRef]














| k | Temperature (°C) | 1/T (K−1) | r2 | Ln k | Activation Energy (kJ/mol) | Half-Life (Months) | Half-Life (Years) |
|---|---|---|---|---|---|---|---|
| 0.003624 | 4 | 0.00361 | 0.3330 | −5.62018 | 34,670.151 | 38.48 | 3.2 |
| 0.01247 | 30 | 0.00330 | 0.8925 | −4.38443 | 11.18 | 0.9 | |
| 0.02095 | 40 | 0.00320 | 0.9591 | −3.86562 | 6.66 | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chittasupho, C.; Tadtong, S.; Vorarat, S.; Imaram, W.; Athikomkulchai, S.; Samee, W.; Sareedenchai, V.; Thongnopkoon, T.; Okonogi, S.; Kamkaen, N. Development of Jelly Loaded with Nanogel Containing Natural L-Dopa from Mucuna pruriens Seed Extract for Neuroprotection in Parkinson’s Disease. Pharmaceutics 2022, 14, 1079. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14051079
Chittasupho C, Tadtong S, Vorarat S, Imaram W, Athikomkulchai S, Samee W, Sareedenchai V, Thongnopkoon T, Okonogi S, Kamkaen N. Development of Jelly Loaded with Nanogel Containing Natural L-Dopa from Mucuna pruriens Seed Extract for Neuroprotection in Parkinson’s Disease. Pharmaceutics. 2022; 14(5):1079. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14051079
Chicago/Turabian StyleChittasupho, Chuda, Sarin Tadtong, Suwanna Vorarat, Witcha Imaram, Sirivan Athikomkulchai, Weerasak Samee, Vipaporn Sareedenchai, Thanu Thongnopkoon, Siriporn Okonogi, and Narisa Kamkaen. 2022. "Development of Jelly Loaded with Nanogel Containing Natural L-Dopa from Mucuna pruriens Seed Extract for Neuroprotection in Parkinson’s Disease" Pharmaceutics 14, no. 5: 1079. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14051079