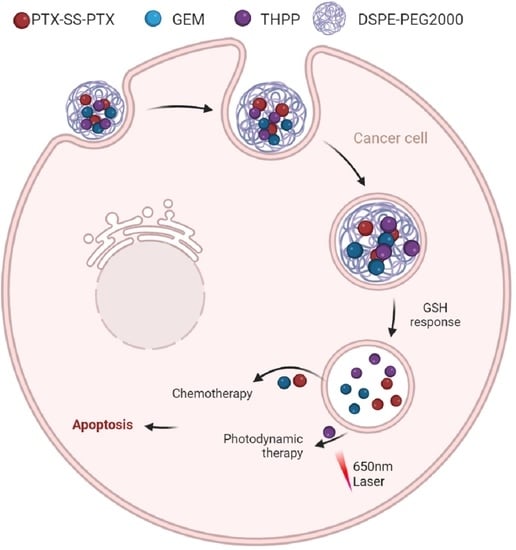
Co-Delivery of Paclitaxel Prodrug, Gemcitabine and Porphine by Micelles for Pancreatic Cancer Treatment via Chemo-Photodynamic Combination Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines and Cell Culture
2.3. TPG Nanoparticles Preparation and In Vitro Release of PTX
2.4. ROS Generation Ability Assay
2.5. Cellular Uptake Assay
2.6. Cellular Lysosomal Escape Assay
2.7. In Vivo Anti-Tumor Therapy
2.8. In Vivo Fluorescence Imaging
3. Results
3.1. PTX-SS-PTX, TPG NPs Synthesis and Characterization
3.2. TPG NPs Cellular Uptake
3.3. TPG NPs In Vitro Cytotoxicity
3.4. Lysosomal Escape of TPG NPs
3.5. ROS Generation in PANC-1 Cells
3.6. Biodistribution of TPG NPs In Vivo
3.7. Anti-Tumor Activity and Biosafety In Vivo
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gentiluomo, M.; Canzian, F.; Nicolini, A.; Gemignani, F.; Landi, S.; Campa, D. Germline genetic variability in pancreatic cancer risk and prognosis. Semin. Cancer Biol. 2022, 79, 105–131. [Google Scholar] [CrossRef] [PubMed]
- Meneses-Medina, M.; Gervaso, L.; Cella, C.; Pellicori, S.; Gandini, S.; Sousa, M.; Fazio, N. Chemotherapy in pancreatic ductal adenocarcinoma: When cytoreduction is the aim. A systematic review and meta-analysis. Cancer Treat. Rev. 2022, 104, 102338. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2020, 70, 313, Erratum in CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tempero, M.; Malafa, M.; Al-Hawary, M.; Behrman, S.; Benson, A.; Cardin, D.; Chiorean, E.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2021, 19, 439–457. [Google Scholar] [CrossRef]
- Xu, F.; Huang, M.; Chen, Q.; Niu, Y.; Hu, Y.; Hu, P.; Chen, D.; He, C.; Huang, K.; Zeng, Z.; et al. LncRNA HIF1A-AS1 Promotes Gemcitabine Resistance of Pancreatic Cancer by Enhancing Glycolysis through Modulating the AKT/YB1/HIF1α Pathway. Cancer Res. 2021, 81, 5678–5691. [Google Scholar] [CrossRef]
- Cui, J.; Guo, Y.; Wu, H.; Xiong, J.; Peng, T. Everolimus regulates the activity of gemcitabine-resistant pancreatic cancer cells by targeting the Warburg effect via PI3K/AKT/mTOR signaling. Mol. Med. 2021, 27, 38. [Google Scholar] [CrossRef]
- Von Hoff, D.; Ervin, T.; Arena, F.; Chiorean, E.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.; Ma, W.; Saleh, M.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [Green Version]
- Blomstrand, H.; Scheibling, U.; Bratthäll, C.; Green, H.; Elander, N. Real world evidence on gemcitabine and nab-paclitaxel combination chemotherapy in advanced pancreatic cancer. BMC Cancer 2019, 19, 40. [Google Scholar] [CrossRef]
- Viúdez, A.; Ramírez, N.; Hernández-García, I.; Carvalho, F.; Vera, R.; Hidalgo, M. Nab-paclitaxel: A flattering facelift. Crit. Rev. Oncol. Hematol. 2014, 92, 166–180. [Google Scholar] [CrossRef]
- Meng, H.; Wang, M.; Liu, H.; Liu, X.; Situ, A.; Wu, B.; Ji, Z.; Chang, C.; Nel, A. Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS Nano 2015, 9, 3540–3557. [Google Scholar] [CrossRef]
- Asrorov, A.; Gu, Z.; Li, F.; Liu, L.; Huang, Y. Biomimetic camouflage delivery strategies for cancer therapy. Nanoscale 2021, 13, 8693–8706. [Google Scholar] [CrossRef] [PubMed]
- Bildstein, L.; Dubernet, C.; Couvreur, P. Prodrug-based intracellular delivery of anticancer agents. Adv. Drug Deliv. Rev. 2011, 63, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Rautio, J.; Meanwell, N.; Di, L.; Hageman, M. The expanding role of prodrugs in contemporary drug design and development. Nat. Rev. Drug Discov. 2018, 17, 559–587. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, H.; Zhang, R.; Zhang, H.; Huang, W. Nanoparticulation of Prodrug into Medicines for Cancer Therapy. Adv. Sci. 2021, 8, e2101454. [Google Scholar] [CrossRef] [PubMed]
- Solmonson, A.; DeBerardinis, R.J. Lipoic acid metabolism and mitochondrial redox regulation. J. Biol. Chem. 2018, 293, 7522–7530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, S.; Rempson, C.M.; Puche, V.; Zhao, B.; Zhang, F. Construction of disulfide containing redox-responsive polymeric nanomedicine. Methods 2022, 199, 67–79. [Google Scholar] [CrossRef]
- Geng, W.; Sessler, J.; Guo, D. Supramolecular prodrugs based on host-guest interactions. Chem. Soc. Rev. 2020, 49, 2303–2315. [Google Scholar] [CrossRef]
- Kakwere, H.; Ingham, E.; Tumbale, S.; Ferrara, K. Gemcitabine-retinoid prodrug loaded nanoparticles display in vitro antitumor efficacy towards drug-resilient human PANC-1 pancreatic cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111251. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, Y.; He, X.; Yu, Y.; Han, R.; Li, Y.; Yang, C.; Hu, D.; Qian, Z. Polymeric Nanoparticles with ROS-Responsive Prodrug and Platinum Nanozyme for Enhanced Chemophotodynamic Therapy of Colon Cancer. Adv. Sci. 2020, 7, 2001853. [Google Scholar] [CrossRef]
- Zhao, H.; Li, L.; Li, F.; Liu, C.; Huang, M.; Li, J.; Gao, F.; Ruan, X.; Yang, D. An Energy-Storing DNA-Based Nanocomplex for Laser-Free Photodynamic Therapy. Adv. Mater. 2022, 34, e2109920. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Wang, X.; Guan, X.; Zhang, W.; Ma, J. Recent advances in selective photothermal therapy of tumor. J. Nanobiotechnol. 2021, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhang, Q.; Sun, B.; Chu, X.; Zhang, M.; She, Z.; Li, Z.; Zhou, N.; Wang, J.; Li, A. MoO nanosheets-based platform for single NIR laser induced efficient PDT/PTT of cancer. J. Control. Release Off. J. Control. Release Soc. 2021, 338, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Choi, K. Advances in Nanomaterial-Mediated Photothermal Cancer Therapies: Toward Clinical Applications. Biomedicines 2021, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, S.; Shi, L.; Teh, C.; Qi, G.; Liu, B. Cancer-Cell-Activated in situ Synthesis of Mitochondria-Targeting AIE Photosensitizer for Precise Photodynamic Therapy. Angew. Chem. 2021, 60, 14945–14953. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, S.; Shao, C.; Acheampong, A.; Khalifa, M.; Liu, C.; Huang, Q. Caenorhabditis elegansGreen synthesis of broccoli-derived carbon quantum dots as effective photosensitizers for the PDT effect testified in the model of mutant. Biomater. Sci. 2022, 10, 2857–2864. [Google Scholar] [CrossRef]
- Nie, W.; Wang, B.; Mi, X.; Chen, J.; Yu, T.; Miao, J.; Lin, Y.; Yang, T.; Ran, M.; Hong, Z.; et al. Co-Delivery of Paclitaxel and shMCL-1 by Folic Acid-Modified Nonviral Vector to Overcome Cancer Chemotherapy Resistance. Small Methods 2021, 5, e2001132. [Google Scholar] [CrossRef]
- Binenbaum, Y.; Na’ara, S.; Gil, Z. Gemcitabine resistance in pancreatic ductal adenocarcinoma. Drug Resist. Updates 2015, 23, 55–68. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, M.; Shi, Y.; Fang, L.; Cao, F. Rational design of mixed nanomicelle eye drops with structural integrity investigation. Acta Biomater. 2022, 141, 164–177. [Google Scholar] [CrossRef]
- Pei, Q.; Hu, X.; Zhou, J.; Liu, S.; Xie, Z. Glutathione-responsive paclitaxel dimer nanovesicles with high drug content. Biomater. Sci. 2017, 5, 1517–1521. [Google Scholar] [CrossRef]
- Tian, X.; Bera, H.; Guo, X.; Xu, R.; Sun, J.; He, Z.; Cun, D.; Yang, M. Pulmonary Delivery of Reactive Oxygen Species/Glutathione-Responsive Paclitaxel Dimeric Nanoparticles Improved Therapeutic Indices against Metastatic Lung Cancer. ACS Appl. Mater. Interfaces 2021, 13, 56858–56872. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Li, Z.; Chen, L.; Xie, Z.; Jing, X. Self-Assembly of Porphyrin-Paclitaxel Conjugates Into Nanomedicines: Enhanced Cytotoxicity due to Endosomal Escape. Chem. Asian J. 2016, 11, 1780–1784. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Okeke, C.I.; Hu, Z.B.; Xu, J. DSPE-PEG: A distinctive component in drug delivery system. Curr. Pharm. Des. 2015, 21, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Selby, L.I.; Johnston, A.P.R.; Such, G.K. The Endosomal Escape of Nanoparticles: Toward More Efficient Cellular Delivery. Bioconjug. Chem. 2019, 30, 263–272. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Ma, X.; Zhou, W.; Yu, R.; Rosenholm, J.M.; Tian, W.; Zhang, L.; Wang, D.; Zhang, H. Co-Delivery of Paclitaxel Prodrug, Gemcitabine and Porphine by Micelles for Pancreatic Cancer Treatment via Chemo-Photodynamic Combination Therapy. Pharmaceutics 2022, 14, 2280. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14112280
Wu Q, Ma X, Zhou W, Yu R, Rosenholm JM, Tian W, Zhang L, Wang D, Zhang H. Co-Delivery of Paclitaxel Prodrug, Gemcitabine and Porphine by Micelles for Pancreatic Cancer Treatment via Chemo-Photodynamic Combination Therapy. Pharmaceutics. 2022; 14(11):2280. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14112280
Chicago/Turabian StyleWu, Qiwei, Xiaodong Ma, Wenhui Zhou, Rong Yu, Jessica M. Rosenholm, Weizhong Tian, Lirong Zhang, Dongqing Wang, and Hongbo Zhang. 2022. "Co-Delivery of Paclitaxel Prodrug, Gemcitabine and Porphine by Micelles for Pancreatic Cancer Treatment via Chemo-Photodynamic Combination Therapy" Pharmaceutics 14, no. 11: 2280. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14112280