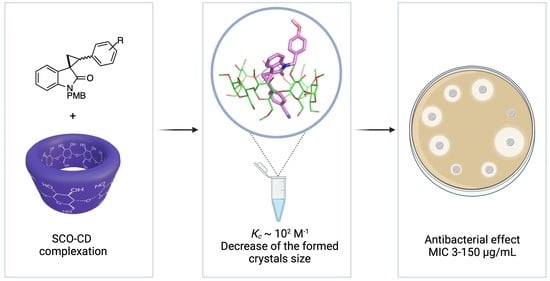
The Solubility Studies and the Complexation Mechanism Investigations of Biologically Active Spiro[cyclopropane-1,3′-oxindoles] with β-Cyclodextrins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Investigated Compounds
General Information
General Procedure for the Synthesis of Alkenes 1
General Procedure for the Synthesis of Cyclopropanes 2
2.3. Measurements
2.3.1. Solubility Studies
2.3.2. UV Spectroscopy
2.3.3. FTIR Microscopy
2.3.4. Dynamic Light Scattering (DLS)
2.3.5. Powder X-ray Diffraction Analysis (PXRD)
2.3.6. Minimum Inhibition Concentration (MIC)
2.3.7. System Preparation
2.3.8. Force-Field Parameterization
2.3.9. System Preparation and Simulation of Molecular Dynamics (MD)
3. Results and Discussion
3.1. Synthesis of SCOs 2
3.2. Solubility of SCOs 2
3.3. Preparation of SCO 2–β-CD Complexes
3.4. Phase Solubility Studies
3.5. Influence of Substituents at β-CDs on SCO Solubility
3.6. Characterization of Inclusion Complexes
3.6.1. PXRD Analysis
3.6.2. FTIR Microscopy
3.6.3. Two-Dimensional NMR Spectroscopy: 1H-1H ROESY Experiments
3.6.4. Molecular Modeling
3.7. Biological Activity of SCOs In Vitro
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Molvi, K.I.; Haque, N.; Awen, B.Z.S.; Zameeruddin, M. ChemInform abstract: Synthesis of spiro compounds as medicinal agents; New opportunities for drug design and discovery. Part I: A review. World J. Pharm. Pharm. Sci. 2014, 3, 536–563. [Google Scholar] [CrossRef]
- Zhou, L.-M.; Qu, R.-Y.; Yang, G.-F. An overview of spirooxindole as a promising scaffold for Novel drug discovery. Expert. Opin. Drug Discov. 2020, 15, 603–625. [Google Scholar] [CrossRef] [PubMed]
- Talele, T.T. The “Cyclopropyl Fragment” is a versatile player that frequently appears in preclinical/clinical drug molecules. J. Med. Chem. 2016, 59, 8712–8756. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?term=CFI-400945&age_v=&gndr=&type=&rslt=&phase=1&phase=2&phase=3&Search=Apply (accessed on 6 November 2022).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?term=RV-521&age_v=&gndr=&type=&rslt=&phase=1&phase=2&phase=3&Search=Apply (accessed on 6 November 2022).
- Jiang, T.; Kuhen, K.L.; Wolff, K.; Yin, H.; Bieza, K.; Caldwell, J.; Bursulaya, B.; Tuntland, T.; Zhang, K.; Karanewsky, D.; et al. Design, synthesis, and biological evaluations of novel oxindoles as HIV-1 non-nucleoside reverse transcriptase inhibitors. Part 2. Bioorg. Med. Chem. Lett. 2006, 16, 2109–2112. [Google Scholar] [CrossRef]
- Hardee, D.; Brewer, J.; Hasvol, L.; Liu, D.; MCDaniel, K.; Schrimpf, M.; Shepard, G. Bromodomain Inhibitors. WO2018188047 A1, 18 October 2018. [Google Scholar]
- Fensome, A.; Mccomas, C.C.; Melensky, E.G.; Marella, M.A.; Wrobel, J.E.; Grubb, G.S. Progesterone Receptor Modulators Comprising Pyrrole-Oxindole Derivates and Uses Thereof. WO2006023109 A1, 2 March 2006. [Google Scholar]
- Beghyn, T.; Deprez, B.; Belouzard, S.; Brodin, P. A Compound As a Thyroid Hormone Beta Receptor Agonist and Use Thereof. WO2021/43185 A1, 11 March 2021. [Google Scholar]
- Chen, L.; Feng, L.; He, Y.; Huang, M.; Yun, H. Spiro Indole-Cyclopropane Indolinones Useful as Ampk Modulators. WO2011070039 A1, 16 June 2011. [Google Scholar]
- Becker, D.P.; Flynn, D.L.; Villamil, C.I. Indolones Useful as Serotonergic Agents. US5399562A, 21 March 1995. [Google Scholar]
- Wurster, J.A. 3-Spyrocyclopropyl2-Oksindole Kinase Inhibitors. WO2007008664 A1, 18 January 2007. [Google Scholar]
- Elder, D.; Holm, R. Aqueous solubility: Simple predictive methods (in silico, in vitro and bio-relevant approaches). Int. J. Pharm. 2013, 453, 3–11. [Google Scholar] [CrossRef]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Duchêne, D.; Bochot, A. Thirty years with cyclodextrins. Int. J. Pharm. 2016, 514, 58–72. [Google Scholar] [CrossRef]
- Uekama, K.; Hirayama, F.; Irie, T. Cyclodextrin drug carrier systems. Chem. Rev. 1998, 98, 2045–2076. [Google Scholar] [CrossRef]
- Gong, L.; Li, T.; Chen, F.; Duan, X.; Yuan, Y.; Zhang, D.; Jiang, Y. An inclusion complex of eugenol into β-Cyclodextrin: Preparation, and physicochemical and antifungal characterization. Food Chem. 2016, 196, 324–330. [Google Scholar] [CrossRef]
- Skuredina, A.A.; Kopnova, T.Y.; Le-Deygen, I.M.; Kudryashova, E.V. Physical and chemical properties of the guest–host inclusion complexes of cyprofloxacin with β-cyclodextrin derivatives. Mosc. Univ. Chem. Bull. 2020, 75, 218–224. [Google Scholar] [CrossRef]
- Řezanka, M. Synthesis of substituted cyclodextrins. Environ. Chem. Lett. 2019, 17, 49–63. [Google Scholar] [CrossRef]
- Corey, E.J.; Chaykovsky, M. Dimethyloxosulfonium methylide ((CH3)2SOCH2) and dimethylsulfonium methylide ((CH3)2SCH2). Formation and application to organic synthesis. J. Am. Chem. Soc. 1965, 87, 1353–1364. [Google Scholar] [CrossRef]
- Fraser, W.; Suckling, C.J.; Wood, H.C.S. Latent Inhibitors. Part 7. Inhibition of dihydro-orotate dehydrogenase by spirocyclopropanobarbiturates. J. Chem. Soc. Perkin 1 1990, 3137. [Google Scholar] [CrossRef]
- Zaytsev, S.V.; Ivanov, K.L.; Skvortsov, D.A.; Bezzubov, S.I.; Melnikov, M.Y.; Budynina, E.M. Nucleophilic ring opening of donor–acceptor cyclopropanes with the cyanate ion: Access to spiro[pyrrolidone-3,3′-oxindoles]. J. Org. Chem. 2018, 83, 8695–8709. [Google Scholar] [CrossRef] [PubMed]
- Baka, E.; Comer, J.E.A.; Takács-Novák, K. Study of equilibrium solubility measurement by saturation shake-flask method using hydrochlorothiazide as model compound. J. Pharm. Biomed. Anal. 2008, 46, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as Pharmaceutical Solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Wang, L.; Yan, J.; Li, Y.; Xu, K.; Li, S.; Tang, P.; Li, H. The influence of hydroxypropyl-β-cyclodextrin on the solubility, dissolution, cytotoxicity, and binding of riluzole with human serum albumin. J. Pharm. Biomed. Anal. 2016, 117, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Kicuntod, J.; Sangpheak, K.; Mueller, M.; Wolschann, P.; Viernstein, H.; Yanaka, S.; Kato, K.; Chavasiri, W.; Pongsawasdi, P.; Kungwan, N.; et al. Theoretical and experimental studies on inclusion complexes of pinostrobin and β-cyclodextrins. Sci. Pharm. 2018, 86, 5. [Google Scholar] [CrossRef] [Green Version]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Roos, K.; Wu, C.; Damm, W.; Reboul, M.; Stevenson, J.M.; Lu, C.; Dahlgren, M.K.; Mondal, S.; Chen, W.; Wang, L.; et al. OPLS3e: Extending force field coverage for drug-like small molecules. J. Chem. Theory Comput. 2019, 15, 1863–1874. [Google Scholar] [CrossRef]
- Gurrath, M.; Müller, G.; Höltje, H.-D. Pseudoreceptor modelling in drug design: Applications of yak and PRGEN. Perspect. Drug Discov. Des. 1998, 12, 135–157. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Junmei Wang, Romain M. Wolf, James W. Caldwell, Peter A. Kollman, and David A. Case, “Development and Testing of a General Amber Force Field” Journal of Computational Chemistry(2004) 25(9) 1157–1174. J. Comput. Chem. 2005, 26, 114. [Google Scholar] [CrossRef]
- Vassetti, D.; Pagliai, M.; Procacci, P. Assessment of GAFF2 and OPLS-AA general force fields in combination with the water models TIP3P, SPCE, and OPC3 for the solvation free energy of druglike organic molecules. J. Chem. Theory Comput. 2019, 15, 1983–1995. [Google Scholar] [CrossRef] [Green Version]
- Jakalian, A.; Bush, B.L.; Jack, D.B.; Bayly, C.I. Fast, efficient generation of high-quality atomic charges. AM1-BCC Model: I. Method. J. Comput. Chem. 2000, 21, 132–146. [Google Scholar] [CrossRef]
- Sousa da Silva, A.W.; Vranken, W.F. ACPYPE–Antechamber python parser interface. BMC Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; van der Spoel, D.; van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Sampson, P.B.; Liu, Y.; Li, S.-W.; Forrest, B.T.; Pauls, H.W.; Edwards, L.G.; Feher, M.; Patel, N.K.B.; Laufer, R. Guohua Pan Kinase Inhibitors and Method of Treating Cancer with Same. WO2011123946 A8, 13 October 2011. [Google Scholar]
- Wang, L.; Li, Z.; Lu, L.; Zhang, W. Synthesis of spiro[furan-3,3′-indolin]-2′-ones by PET-catalyzed [3+2] reactions of spiro[indoline-3,2′-oxiran]-2-ones with electron-Rich olefins. Tetrahedron 2012, 68, 1483–1491. [Google Scholar] [CrossRef]
- Shinada, N.K.; de Brevern, A.G.; Schmidtke, P. Halogens in protein–ligand binding mechanism: A structural perspective. J. Med. Chem. 2019, 62, 9341–9356. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Y.; Huang, N. A survey of the role of nitrile groups in protein–ligand interactions. Future Med. Chem. 2018, 10, 2713–2728. [Google Scholar] [CrossRef]
- Sukhoverkov, K.V.; Le-Deygen, I.M.; Egorov, A.M.; Kudryashova, E.V. Physicochemical properties of the inclusion complex of moxifloxacin with hydroxypropyl-β-cyclodextrin synthesized by RESS. Russ. J. Phys. Chem. B 2018, 12, 1193–1204. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Méndez, S.G.; Otero Espinar, F.J.; Alvarez, A.L.; Longhi, M.R.; Quevedo, M.A.; Zoppi, A. Ternary complexation of benzoic acid with β-cyclodextrin and aminoacids. Experimental and theoretical studies. J. Incl. Phenom. Macrocycl. Chem. 2016, 85, 33–48. [Google Scholar] [CrossRef]
- McIntosh, M.P.; Schwarting, N.; Rajewski, R.A. In vitro and in vivo evaluation of a sulfobutyl ether Β-cyclodextrin Enabled etomidate formulation. J. Pharm. Sci. 2004, 93, 2585–2594. [Google Scholar] [CrossRef]
- del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Crupi, V.; Ficarra, R.; Guardo, M.; Majolino, D.; Stancanelli, R.; Venuti, V. UV–vis and FTIR–ATR spectroscopic techniques to study the inclusion complexes of genistein with β-cyclodextrins. J. Pharm. Biomed. Anal. 2007, 44, 110–117. [Google Scholar] [CrossRef]
- Le-Deygen, I.M.; Skuredina, A.A.; Uporov, I.V.; Kudryashova, E.V. Thermodynamics and molecular insight in guest–host complexes of fluoroquinolones with β-cyclodextrin derivatives, as revealed by ATR-FTIR spectroscopy and molecular modeling experiments. Anal. Bioanal. Chem. 2017, 409, 6451–6462. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Barman, B.K.; Roy, M.N. Preparation, characterization and binding behaviors of host-guest inclusion complexes of metoclopramide hydrochloride with α- and β-cyclodextrin molecules. J. Mol. Struct. 2018, 1155, 503–512. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Kumar, G. NMR and molecular modelling studies on the interaction of fluconazole with β-cyclodextrin. Chem. Cent. J. 2009, 3, 9. [Google Scholar] [CrossRef]
- Loftsson, T.; Moya-Ortega, M.D.; Alvarez-Lorenzo, C.; Concheiro, A. Pharmacokinetics of cyclodextrins and drugs after oral and parenteral administration of drug/cyclodextrin complexes. J. Pharm. Pharmacol. 2016, 68, 544–555. [Google Scholar] [CrossRef]
- Skuredina, A.A.; Tychinina, A.S.; Le-Deygen, I.M.; Golyshev, S.A.; Kopnova, T.Y.; Le, N.T.; Belogurova, N.G.; Kudryashova, E.V. Cyclodextrins and their polymers affect the lipid membrane permeability and increase levofloxacin’s antibacterial activity in vitro. Polymers 2022, 14, 4476. [Google Scholar] [CrossRef]
- Sala, A.; Hoossen, Z.; Bacchi, A.; Caira, M.R. Two crystal forms of a hydrated 2:1 β-cyclodextrin fluconazole complex: Single crystal X-ray structures, dehydration profiles, and conditions for their individual isolation. Molecules 2021, 26, 4427. [Google Scholar] [CrossRef]
- Kohut, A.; Demchuk, Z.; Kingsley, K.; Voronov, S.; Voronov, A. Dual role of methyl-β-cyclodextrin in the emulsion polymerization of highly hydrophobic plant oil-based monomers with various unsaturations. Eur. Polym. J. 2018, 108, 322–328. [Google Scholar] [CrossRef]
- Yang, W.; Johnston, K.P.; Williams, R.O. Comparison of bioavailability of amorphous versus crystalline itraconazole nanoparticles via pulmonary administration in rats. Eur. J. Pharm. Biopharm. 2010, 75, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Skuredina, A.A.; Tychinina, A.S.; Le-Deygen, I.M.; Golyshev, S.A.; Belogurova, N.G.; Kudryashova, E.V. The formation of quasi-regular polymeric network of cross-linked sulfobutyl ether derivative of β-cyclodextrin synthesized with moxifloxacin as a template. React. Funct. Polym. 2021, 159, 104811. [Google Scholar] [CrossRef]
- Skuredina, A.A.; Danilov, M.R.; Le-Deygen, I.M.; Kudryashova, E.V. Adsorption Properties of mesoporous silica gel with β-cyclodextrin as a pore-forming agent relative to moxifloxacin. Mosc. Univ. Chem. Bull. 2018, 73, 192–198. [Google Scholar] [CrossRef]
- Palomba, M.; Rossi, L.; Sancineto, L.; Tramontano, E.; Corona, A.; Bagnoli, L.; Santi, C.; Pannecouque, C.; Tabarrini, O.; Marini, F. A new vinyl selenone-based domino approach to spirocyclopropyl oxindoles endowed with anti-HIV rt activity. Org. Biomol. Chem. 2016, 14, 2015–2024. [Google Scholar] [CrossRef]
- Kavyani, S.; Baharfar, R. Design and characterization of Fe3O4/GO/Au-Ag nanocomposite as an efficient catalyst for the green synthesis of spirooxindole-dihydropyridines. Appl. Organomet. Chem. 2020, 34, e5560. [Google Scholar] [CrossRef]
- Allahresani, A.; Taheri, B.; Nasseri, M.A. A green synthesis of spirooxindole derivatives catalyzed by SiO2@g-C3N4 nanocomposite. Res. Chem. Intermed. 2018, 44, 1173–1188. [Google Scholar] [CrossRef]
- Liang, H.; Yuan, Q.; Vriesekoop, F.; Lv, F. Effects of cyclodextrins on the antimicrobial activity of plant-derived essential oil compounds. Food Chem. 2012, 135, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
















| 2 | Ar | Sa, mg/mL |
|---|---|---|
| a | 2-ClC6H4 | 0.18 ± 0.02 |
| b | 4-NCC6H4 | 0.84 ± 0.03 |
| c | 4-t-BuC6H4 | 0.11 ± 0.02 |
| d | 3,4-(MeO)2C6H3 | 0.27 ± 0.03 |
| 2a | HPβCD-2a | |
|---|---|---|
| C-HAlk | 2935 ± 0.5 | 2935 ± 0.5 |
| 2840 ± 0.5 | 2840 ± 0.5 | |
| Amide I (C=O) | 1697 ± 0.5 | 1701 ± 0.5 |
| Amide II (N-C=O) | 1613 ± 0.5 | 1617 ± 0.5 |
| CAr-H | 1481 ± 0.5 | - |
| C-O-CAr | 1246 ± 0.5 | 1248 ± 0.5 |
| CAr-Cl | 1031 ± 0.5 | 1030 ± 0.5 |
| MIC, μg/mL | CD | 2a | 2b |
|---|---|---|---|
| Without CD | 1000 ± 35 | 3.2 ± 0.3 | |
| HPβCD | − | 150 ± 20 | 3.0 ± 0.4 |
| MβCD | − | 140 ± 23 | 3.4 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kravtsova, A.A.; Skuredina, A.A.; Malyshev, A.S.; Le-Deygen, I.M.; Kudryashova, E.V.; Budynina, E.M. The Solubility Studies and the Complexation Mechanism Investigations of Biologically Active Spiro[cyclopropane-1,3′-oxindoles] with β-Cyclodextrins. Pharmaceutics 2023, 15, 228. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics15010228
Kravtsova AA, Skuredina AA, Malyshev AS, Le-Deygen IM, Kudryashova EV, Budynina EM. The Solubility Studies and the Complexation Mechanism Investigations of Biologically Active Spiro[cyclopropane-1,3′-oxindoles] with β-Cyclodextrins. Pharmaceutics. 2023; 15(1):228. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics15010228
Chicago/Turabian StyleKravtsova, Anna A., Anna A. Skuredina, Alexander S. Malyshev, Irina M. Le-Deygen, Elena V. Kudryashova, and Ekaterina M. Budynina. 2023. "The Solubility Studies and the Complexation Mechanism Investigations of Biologically Active Spiro[cyclopropane-1,3′-oxindoles] with β-Cyclodextrins" Pharmaceutics 15, no. 1: 228. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics15010228