Formulation Approaches to Crystalline Status Modification for Carotenoids: Impacts on Dissolution, Stability, Bioavailability, and Bioactivities
Abstract
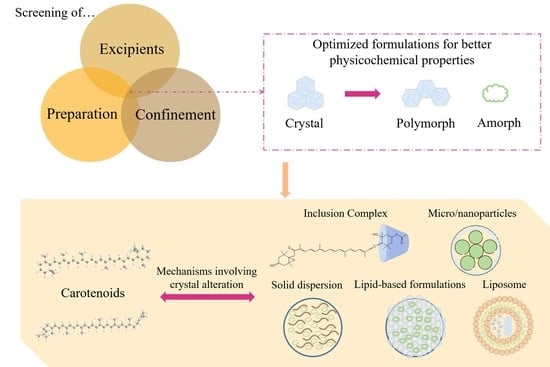
:1. Introduction
| Carotenoids | Dose | Model | Bioactivities | Reference |
|---|---|---|---|---|
| Astaxanthin | 1.0 mg/mouse/day | Diabetic C57BL/KsJ-db/db mice | Anti-diabetic (Blood glucose↓ and preservation of -cell function) | [9] |
| Astaxanthin | 2 mg | Healthy women | Immune response improvement (Mitogen-induced lymphoproliferation↑ Natural killer cell, total T and B cell↑ DNA damage biomarker↓) | [10] |
| Astaxanthin | 5 μM | Primary hippocampal neurons | Treatment of Hcy-mediated neurological disorders (ROS and superoxide anion↓) | [11] |
| β-Carotene | 45 mg/day | Healthy older adults | Immunostimulant (Total T cells and NK cell↑) | [12] |
| β-Carotene | 200 mg/Kg | Male albino mice | Anticonvulsant activity (Duration of general tonic–clonic seizures↓ General tonic–clonic seizures latency↑) | [13] |
| β-Carotene | 30 μM | Human prostate cancer cell line (PC-3 cell) | Anticancer (cell viability: 51.4%) | [14] |
| β-Carotene | 2.05 mg/Kg | Male albino mice | Treatment of Alzheimer’s disease (Acetylcholinesterase and amyloid β-protein↓) | [15] |
| β-Cryptoxanthin | 0.8 mg/Kg/day | Male mice | Anti-obesity (Adipocyte hypertrophy↓) | [16] |
| Fucoxanthin | 5 μM | Human fibroblast | Protection against UVB radiation-induced oxidative stress (ROS↓) | [17] |
| Fucoxanthin | 1.06-2.22% | C57BL/6J mice | Anti-obesity and anti-diabetic effects (Body weight and white adipose tissue↓ MCP-1 expression↓ and Adrb3 and GLUT4↑) | [18] |
| Fucoxanthin | 0.2% | C57BL/6N mice | Anti-obesity (Fatty acid β-oxidation activity and lipogenic enzyme activities ↓) | [19] |
| Lutein and Zeaxanthin | Oral: lutein 100 ppm zeaxanthin 6 ppm Topical: lutein 10 ppm zeaxanthin 0.6 ppm | Healthy women | Photoprotective (Lipid peroxidation↓ skin lipid, skin hydration and skin elasticity↑) | [20] |
| Lutein and Zeaxanthin | Lutein: 5% zeaxanthin: 0.2% | β5−/− mice | Prevention of age-related retinal pigment epithelium actin damage (4-hydroxynonenal-adduct formation, age-related cone and rod photoreceptor dysfunction ↓) | [21] |
| Lutein and Zeaxanthin | Lutein:10 mg Zeaxanthin: 2 mg | Healthy older adults | Improvement of cognitive function (Macular pigment optical density, complex attention and cognitive flexibility domains↑) | [22] |
| Lycopene | 5 μg/mL | Fungal cell (Candida albicans) | Antifungi (Destruction of fungi membrane and inhibition of the normal budding process) | [23] |
| Lycopene | 2 μM | Rat cortical neurons | Treatment of Alzheimer’s disease (Intracellular ROS and superoxide production↓) | [24] |
| Lycopene | 0.2 or 0.5 μM | Neuronal SH-SY5Y cells | Neuron protection (ROS↓ and mitochondrial dysfunction ↓) | [25] |
| Lycopene | 0.03% (w/w, mixed into normal chow) | Male C57BL/6J mice | Treatment of Alzheimer’s disease (Memory loss behavior, amyloid plaques, amyloid precursor protein, neuronal β-secretase BACE1, inflammatory mediators and oxidative stress↓, α-secretase ADAM10↑) | [26] |
| Lycopene | 2 μM | Mice cerebral cortical neurons | Neuron protection (Nerve growth factor, brain-derived neurotrophic factor, and vascular endothelial growth factor excretion↑ and anti-apoptosis) | [27] |
| Lycopene | 100 mg/Kg | Female Sprague-Dawley rats | Treatment of vascular dementia (Oxidative stress in hippocampus↓) | [28] |
2. Effects of Crystalline Status Modification on the Physicochemical Properties of Carotenoids
3. Methods for Examining the Crystalline Status
3.1. PXRD
3.2. Electron Microscopy—TEM and SEM
3.3. Thermal Methods—DSC and TGA
4. Effects of Preparation Factors on Crystalline Status of Active Pharmaceutical Ingredients
4.1. Excipients
4.2. Preparation
4.3. Confinement (Change in Particle Sizes)
5. Approaches for the Crystalline Status Modification of Carotenoids
5.1. Co-Crystallization
5.2. Solid Dispersion
5.3. Inclusion Complex
5.4. Micro/Nano Particles
5.5. Lipid-Based Formulations
5.5.1. Solid Lipid Nanoparticles/Microparticles
5.5.2. Nanostructured Lipid Carrier
5.5.3. Microemulsion/Nanoemulsion
5.5.4. Self-Emulsifying Drug Delivery System
5.6. Liposome
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gary Williamson, A.G.M.; Graham, A.B.; Catherine, S.B. Food Chemistry, Function and Analysis; Gary Williamson, A.G.M., Graham, A.B., Catherine, S.B., Eds.; RSC Publishing: Cambridge, UK, 2019; pp. 15–20. [Google Scholar] [CrossRef]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.-H. Recent advances on nanoparticle based strategies for improving carotenoid stability and biological activity. Antioxidants 2021, 10, 713. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta 2005, 1740, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Bayr, H. Reactive oxygen species. Crit. Care Med. 2005, 33, S498–S501. [Google Scholar] [CrossRef]
- Adejare, A. Remington: The Science and Practice of Pharmacy; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Honda, M.; Kageyama, H.; Hibino, T.; Zhang, Y.; Diono, W.; Kanda, H.; Yamaguchi, R.; Takemura, R.; Fukaya, T.; Goto, M. Improved carotenoid processing with sustainable solvents utilizing Z-isomerization-induced alteration in physicochemical properties: A review and future directions. Molecules 2019, 24, 2149. [Google Scholar] [CrossRef] [PubMed]
- Stegemann, S.; Leveiller, F.; Franchi, D.; De Jong, H.; Lindén, H. When poor solubility becomes an issue: From early stage to proof of concept. Eur. J. Pharm. Sci. 2007, 31, 249–261. [Google Scholar] [CrossRef]
- Van den Mooter, G. The use of amorphous solid dispersions: A formulation strategy to overcome poor solubility and dissolution rate. Drug Discov. Today Technol. 2012, 9, e79–e85. [Google Scholar] [CrossRef]
- Uchiyama, K.; Naito, Y.; Hasegawa, G.; Nakamura, N.; Takahashi, J.; Yoshikawa, T. Astaxanthin protects β-cells against glucose toxicity in diabetic db/db mice. Redox Rep. 2002, 7, 290–293. [Google Scholar] [CrossRef]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 1–10. [Google Scholar] [CrossRef]
- Wang, X.-j.; Chen, W.; Fu, X.-t.; Ma, J.-k.; Wang, M.-h.; Hou, Y.-j.; Tian, D.-c.; Fu, X.-y.; Fan, C.-d. Reversal of homocysteine-induced neurotoxicity in rat hippocampal neurons by astaxanthin: Evidences for mitochondrial dysfunction and signaling crosstalk. Cell Death Discov. 2018, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wood, S.M.; Beckham, C.; Yosioka, A.; Darban, H.; Watson, R.R. β-Carotene and selenium supplementation enhances immune response in aged humans. Integr. Med. Int. 2000, 2, 85–92. [Google Scholar] [CrossRef]
- Yusuf, M.; Khan, R.A.; Khan, M.; Ahmed, B. Plausible antioxidant biomechanics and anticonvulsant pharmacological activity of brain-targeted β-carotene nanoparticles. Int. J. Nanomed. 2012, 7, 4311. [Google Scholar]
- Jayappriyan, K.; Rajkumar, R.; Venkatakrishnan, V.; Nagaraj, S.; Rengasamy, R. In vitro anticancer activity of natural β-carotene from Dunaliella salina EU5891199 in PC-3 cells. Biomed. Prev. Nutr. 2013, 3, 99–105. [Google Scholar] [CrossRef]
- Hira, S.; Saleem, U.; Anwar, F.; Sohail, M.F.; Raza, Z.; Ahmad, B. β-Carotene: A natural compound improves cognitive impairment and oxidative stress in a mouse model of streptozotocin-induced Alzheimer’s disease. Biomolecules 2019, 9, 441. [Google Scholar] [CrossRef]
- Takayanagi, K.; Morimoto, S.-i.; Shirakura, Y.; Mukai, K.; Sugiyama, T.; Tokuji, Y.; Ohnishi, M. Mechanism of visceral fat reduction in Tsumura Suzuki obese, diabetes (TSOD) mice orally administered β-cryptoxanthin from Satsuma mandarin oranges (Citrus unshiu Marc). J. Agric. Food Chem. 2011, 59, 12342–12351. [Google Scholar] [CrossRef]
- Heo, S.-J.; Jeon, Y.-J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B Biol. 2009, 95, 101–107. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Murakami-Funayama, K.; Miyashita, K. Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol. Med. Rep. 2009, 2, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.N.; Jeon, S.M.; Shin, Y.C.; Lee, M.K.; Kang, M.A.; Choi, M.S. Anti-obese property of fucoxanthin is partly mediated by altering lipid-regulating enzymes and uncoupling proteins of visceral adipose tissue in mice. Mol. Nutr. Food Res. 2009, 53, 1603–1611. [Google Scholar] [CrossRef]
- Palombo, P.; Fabrizi, G.; Ruocco, V.; Ruocco, E.; Fluhr, J.; Roberts, R.; Morganti, P. Beneficial long-term effects of combined oral/topical antioxidant treatment with the carotenoids lutein and zeaxanthin on human skin: A double-blind, placebo-controlled study. Ski. Pharmacol. Physiol. 2007, 20, 199–210. [Google Scholar] [CrossRef]
- Yu, C.-C.; Nandrot, E.F.; Dun, Y.; Finnemann, S.C. Dietary antioxidants prevent age-related retinal pigment epithelium actin damage and blindness in mice lacking αvβ5 integrin. Free Radic. Biol. Med. 2012, 52, 660–670. [Google Scholar] [CrossRef]
- Hammond Jr, B.R.; Miller, L.S.; Bello, M.O.; Lindbergh, C.A.; Mewborn, C.; Renzi-Hammond, L.M. Effects of lutein/zeaxanthin supplementation on the cognitive function of community dwelling older adults: A randomized, double-masked, placebo-controlled trial. Front. Aging Neurosci. 2017, 9, 254. [Google Scholar] [CrossRef]
- Sung, W.-S.; Lee, I.-S.; Lee, D.-G. Damage to the cytoplasmic membrane and cell death caused by lycopene in Candida albicans. J. Microbiol. Biotechnol. 2007, 17, 1797–1804. [Google Scholar]
- Qu, M.; Jiang, Z.; Liao, Y.; Song, Z.; Nan, X. Lycopene prevents amyloid [beta]-induced mitochondrial oxidative stress and dysfunctions in cultured rat cortical neurons. Neurochem. Res. 2016, 41, 1354–1364. [Google Scholar] [CrossRef]
- Hwang, S.; Lim, J.W.; Kim, H. Inhibitory effect of lycopene on amyloid-β-induced apoptosis in neuronal cells. Nutrients 2017, 9, 883. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, L.; Wang, Z.; Cui, Y.; Tan, X.; Yuan, T.; Liu, Q.; Liu, Z.; Liu, X. Supplementation of lycopene attenuates lipopolysaccharide-induced amyloidogenesis and cognitive impairments via mediating neuroinflammation and oxidative stress. J. Nutr. Biochem. 2018, 56, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Gan, D.; Fan, C.; Wen, C.; Li, A.; Li, Q.; Zhao, J.; Wang, Z.; Zhu, L.; Lu, D. The secretion from neural stem cells pretreated with lycopene protects against tert-butyl hydroperoxide-induced neuron oxidative damage. Oxid. Med. Cell. Longev. 2018, 2018, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.-W.; Yin, X.-L.; Lin, R.; Fan, X.-L.; Chen, S.-J.; Zhu, Y.-M.; Zhao, X.-Z. Possible mechanisms of lycopene amelioration of learning and memory impairment in rats with vascular dementia. Neural Regen. Res. 2020, 15, 332. [Google Scholar] [CrossRef]
- Chasse, G.A.; Chasse, K.P.; Kucsman, A.; Torday, L.L.; Papp, J.G. Conformational potential energy surfaces of a lycopene model. J. Mol. Struct.: THEOCHEM 2001, 571, 7–26. [Google Scholar] [CrossRef]
- Khoo, H.-E.; Prasad, K.N.; Kong, K.-W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Dewanjee, S.; Riaz, M. Carotenoids: Structure and Function in the Human Body; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Jing, C.; Qun, X.; Rohrer, J. HPLC separation of all-trans-β-carotene and its iodine-induced isomers using a C30 column. Thermo Sci. 2012, 391, 2–6. [Google Scholar]
- Yang, C.; Fischer, M.; Kirby, C.; Liu, R.; Zhu, H.; Zhang, H.; Chen, Y.; Sun, Y.; Zhang, L.; Tsao, R. Bioaccessibility, cellular uptake and transport of luteins and assessment of their antioxidant activities. Food Chem. 2018, 249, 66–76. [Google Scholar] [CrossRef]
- Yang, C.; Hassan, Y.I.; Liu, R.; Zhang, H.; Chen, Y.; Zhang, L.; Tsao, R. Anti-inflammatory effects of different astaxanthin isomers and the roles of lipid transporters in the cellular transport of astaxanthin isomers in Caco-2 cell monolayers. J. Agric. Food Chem. 2019, 67, 6222–6231. [Google Scholar] [CrossRef] [PubMed]
- During, A.; Hussain, M.M.; Morel, D.W.; Harrison, E.H. Carotenoid uptake and secretion by CaCo-2 cells: β-carotene isomer selectivity and carotenoid interactions1. J. Lipid Res. 2002, 43, 1086–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furr, H.C.; Clark, R.M. Intestinal absorption and tissue distribution of carotenoids. J. Nutr. Biochem. 1997, 8, 364–377. [Google Scholar] [CrossRef]
- Sato, H.; Matsushita, T.; Ueno, K.; Kikuchi, H.; Yamada, K.; Onoue, S. Oily Suspension of Micronized Crystalline Lutein for the Improvement of Dissolution and Oral Bioavailability. Food Sci. Technol. 2022, 2, 272–279. [Google Scholar] [CrossRef]
- Faller, B.; Desrayaud, S.; Berghausen, J.; Laisney, M.; Dodd, S. 4 How solubility influences bioavailability. Solubility Pharm. Chem. 2020, 113, 113–132. [Google Scholar]
- Pudipeddi, M.; Serajuddin, A.T. Trends in solubility of polymorphs. J. Pharm. Sci. 2005, 94, 929–939. [Google Scholar] [CrossRef]
- Kaushal, A.M.; Gupta, P.; Bansal, A.K. Amorphous drug delivery systems: Molecular aspects, design, and performance. Crit. Rev. Ther. Drug Carr. Syst. 2004, 21, 133–193. [Google Scholar] [CrossRef]
- Vasconcelos, T.; Marques, S.; das Neves, J.; Sarmento, B. Amorphous solid dispersions: Rational selection of a manufacturing process. Adv. Drug. Deliv. Rev. 2016, 100, 85–101. [Google Scholar] [CrossRef]
- Healy, A.M.; Worku, Z.A.; Kumar, D.; Madi, A.M. Pharmaceutical solvates, hydrates and amorphous forms: A special emphasis on cocrystals. Adv. Drug. Deliv. Rev. 2017, 117, 25–46. [Google Scholar] [CrossRef]
- Zhou, W.; Greer, H.F. What can electron microscopy tell us beyond crystal structures? Eur. J. Inorg. Chem. 2016, 2016, 941–950. [Google Scholar] [CrossRef]
- Stevenson, H.P.; Makhov, A.M.; Calero, M.; Edwards, A.L.; Zeldin, O.B.; Mathews, I.I.; Lin, G.; Barnes, C.O.; Santamaria, H.; Ross, T.M. Use of transmission electron microscopy to identify nanocrystals of challenging protein targets. Proc. Natl. Acad. Sci. USA 2014, 111, 8470–8475. [Google Scholar] [CrossRef]
- Nurit, T.-G. Minerals Observed by Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM) and High Resolution Transmission Electron Microscopy (HRTEM). In Electron Microscopy; IntechOpen: London, UK, 2022. [Google Scholar]
- Bruni, G.; Berbenni, V.; Sartor, F.; Milanese, C.; Girella, A.; Franchi, D.; Marini, A. Quantification methods of amorphous/crystalline fractions in high-energy ball milled pharmaceutical products. J. Therm. Anal. Calorim. 2012, 108, 235–241. [Google Scholar] [CrossRef]
- Leyva-Porras, C.; Cruz-Alcantar, P.; Espinosa-Solís, V.; Martínez-Guerra, E.; Piñón-Balderrama, C.I.; Compean Martínez, I.; Saavedra-Leos, M.Z. Application of differential scanning calorimetry (DSC) and modulated differential scanning calorimetry (MDSC) in food and drug industries. Polymers 2019, 12, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.G.; Branford-White, C.; Li, L.; Wu, X.M.; Zhu, L.M. The compatibility of acyclovir with polyacrylonitrile in the electrospun drug-loaded nanofibers. J. Appl. Polym. Sci. 2010, 117, 1509–1515. [Google Scholar] [CrossRef]
- Shah, B.; Kakumanu, V.K.; Bansal, A.K. Analytical techniques for quantification of amorphous/crystalline phases in pharmaceutical solids. J. Pharm. Sci. 2006, 95, 1641–1665. [Google Scholar] [CrossRef]
- Kumar, S.; Rao, R. Analytical tools for cyclodextrin nanosponges in pharmaceutical field: A review. J. Incl. Phenom. Macrocycl. Chem. 2019, 94, 11–30. [Google Scholar] [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in the solid state: A review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Wu, H.; Cai, T. Effect of polymeric excipients on nucleation and crystal growth kinetics of amorphous fluconazole. Biomater. Sci. 2021, 9, 4308–4316. [Google Scholar] [CrossRef]
- Wilson, V.R.; Lou, X.; Osterling, D.J.; Stolarik, D.F.; Jenkins, G.J.; Nichols, B.L.; Dong, Y.; Edgar, K.J.; Zhang, G.G.; Taylor, L.S. Amorphous solid dispersions of enzalutamide and novel polysaccharide derivatives: Investigation of relationships between polymer structure and performance. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Bayard, M.; Cansell, M.; Leal-Calderon, F. Crystallization of emulsified anhydrous milk fat: The role of confinement and of minor compounds. A DSC Study. Food Chem. 2022, 373, 131605. [Google Scholar] [CrossRef]
- Bayard, M.; Kauffmann, B.; Vauvre, J.-M.; Leal-Calderon, F.; Cansell, M. Isothermal crystallization of anhydrous milk fat in presence of free fatty acids and their esters: From nanostructure to textural properties. Food Chem. 2022, 366, 130533. [Google Scholar] [CrossRef] [PubMed]
- Gift, A.D.; Southard, L.A.; Riesberg, A.L. Influence of polymeric excipient properties on crystal hydrate formation kinetics of caffeine in aqueous slurries. J. Pharm. Sci. 2012, 101, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Teja, S.B.; Patil, S.P.; Shete, G.; Patel, S.; Bansal, A.K. Drug-excipient behavior in polymeric amorphous solid dispersions. J. Excip. Food Chem. 2016, 4, 1048. [Google Scholar]
- Iyer, R.; Petrovska Jovanovska, V.; Berginc, K.; Jaklič, M.; Fabiani, F.; Harlacher, C.; Huzjak, T.; Sanchez-Felix, M.V. Amorphous Solid Dispersions (ASDs): The Influence of Material Properties, Manufacturing Processes and Analytical Technologies in Drug Product Development. Pharmaceutics 2021, 13, 1682. [Google Scholar] [CrossRef] [PubMed]
- Einfalt, T.; Planinšek, O.; Hrovat, K. Methods of amorphization and investigation of the amorphous state. Acta Pharm. 2013, 63, 305–334. [Google Scholar] [CrossRef] [PubMed]
- Noonan, T.J.; Chibale, K.; Cheuka, P.M.; Kumar, M.; Bourne, S.A.; Caira, M.R. Five solid forms of a potent imidazopyridazine antimalarial drug lead: A preformulation study. Cryst. Growth Des. 2019, 19, 4683–4697. [Google Scholar] [CrossRef]
- Diao, Y.; Whaley, K.E.; Helgeson, M.E.; Woldeyes, M.A.; Doyle, P.S.; Myerson, A.S.; Hatton, T.A.; Trout, B.L. Gel-induced selective crystallization of polymorphs. J. Am. Chem. Soc. 2012, 134, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Beiner, M.; Rengarajan, G.; Pankaj, S.; Enke, D.; Steinhart, M. Manipulating the crystalline state of pharmaceuticals by nanoconfinement. Nano Lett. 2007, 7, 1381–1385. [Google Scholar] [CrossRef]
- Cheng, S.; McKenna, G.B. Nanoconfinement effects on the glass transition and crystallization behaviors of nifedipine. Mol. Pharm. 2019, 16, 856–866. [Google Scholar] [CrossRef]
- Rengarajan, G.; Enke, D.; Steinhart, M.; Beiner, M. Stabilization of the amorphous state of pharmaceuticals in nanopores. J. Mater. Chem. 2008, 18, 2537–2539. [Google Scholar] [CrossRef]
- Cipolla, D.; Wu, H.; Salentinig, S.; Boyd, B.; Rades, T.; Vanhecke, D.; Petri-Fink, A.; Rothin-Rutishauser, B.; Eastman, S.; Redelmeier, T. Formation of drug nanocrystals under nanoconfinement afforded by liposomes. RSC Adv. 2016, 6, 6223–6233. [Google Scholar] [CrossRef]
- Salas-Zúñiga, R.; Mondragón-Vásquez, K.; Alcalá-Alcalá, S.; Lima, E.; Höpfl, H.; Herrera-Ruiz, D.; Morales-Rojas, H. Nanoconfinement of a Pharmaceutical Cocrystal with Praziquantel in Mesoporous Silica: The Influence of the Solid Form on Dissolution Enhancement. Mol. Pharm. 2021, 19, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Salmon, D.J. Building co-crystals with molecular sense and supramolecular sensibility. CrystEngComm 2005, 7, 439–448. [Google Scholar] [CrossRef]
- Steed, J.W. The role of co-crystals in pharmaceutical design. Trends Pharmacol. Sci. 2013, 34, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miroshnyk, I.; Mirza, S.; Sandler, N. Pharmaceutical co-crystals–an opportunity for drug product enhancement. Expert Opin. Drug Deliv. 2009, 6, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Elsayed, A.; Subramanian, J.; Singh, A. Encapsulation of carotenoids with sucrose by co-crystallization: Physicochemical properties, characterization and thermal stability of pigments. LWT 2021, 140, 110810. [Google Scholar] [CrossRef]
- Chezanoglou, E.; Goula, A.M. Co-crystallization in sucrose: A promising method for encapsulation of food bioactive components. Trends Food Sci. Technol. 2021, 114, 262–274. [Google Scholar] [CrossRef]
- Good, D.J.; Rodriguez-Hornedo, N. Solubility advantage of pharmaceutical cocrystals. Cryst. Growth Des. 2009, 9, 2252–2264. [Google Scholar] [CrossRef]
- Huang, Y.; Dai, W.-G. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharm. Sin. B. 2014, 4, 18–25. [Google Scholar] [CrossRef]
- Newman, A.; Knipp, G.; Zografi, G. Assessing the performance of amorphous solid dispersions. J. Pharm. Sci. 2012, 101, 1355–1377. [Google Scholar] [CrossRef]
- Ishimoto, K.; Miki, S.; Ohno, A.; Nakamura, Y.; Otani, S.; Nakamura, M.; Nakagawa, S. β-Carotene solid dispersion prepared by hot-melt technology improves its solubility in water. J. Food Sci. Technol. 2019, 56, 3540–3546. [Google Scholar] [CrossRef] [PubMed]
- Otani, S.; Miki, S.; Nakamura, Y.; Ishimoto, K.; Ago, Y.; Nakagawa, S. Improved bioavailability of β-carotene by amorphous solid dispersion technology in rats. J. Nutr. Sci. Vitaminol. 2020, 66, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, K.; Nakamura, Y.; Otani, S.; Miki, S.; Maeda, S.; Iwamoto, T.; Konishi, Y.; Ago, Y.; Nakagawa, S. Examination of dissolution ratio of β-carotene in water for practical application of β-carotene amorphous solid dispersion. J. Food Sci. Technol. 2022, 59, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, C.; Hu, X.; Campanella, O.H.; Miao, M. Fabrication and characterizations of cyclic amylopectin-based delivery system incorporated with β-carotene. Food Hydrocoll. 2022, 130, 107680. [Google Scholar] [CrossRef]
- Chang, C.-W.; Wang, C.-Y.; Wu, Y.-T.; Hsu, M.-C. Enhanced solubility, dissolution, and absorption of lycopene by a solid dispersion technique: The dripping pill delivery system. Powder Technol. 2016, 301, 641–648. [Google Scholar] [CrossRef]
- Fuenmayor, C.A.; Baron-Cangrejo, O.G.; Salgado-Rivera, P.A. Encapsulation of carotenoids as food colorants via formation of cyclodextrin inclusion complexes: A review. Polysaccharides 2021, 2, 454–476. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, J.; Liu, C.; Wang, D.; Wang, C.-Y. Fabrication of fucoxanthin/2-hydroxypropyl-β-cyclodextrin inclusion complex assisted by ultrasound procedure to enhance aqueous solubility, stability and antitumor effect of fucoxanthin. Ultrason. Sonochem. 2022, 90, 106215. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Puentes, A.A.; Reyes-López, S.Y.; Baltazar, Á.d.J.R.; López-Teros, V.; Wall-Medrano, A. Molecular interaction of β-carotene with sweet potato starch: A bleaching-restitution assay. Food Hydrocoll. 2022, 127, 107522. [Google Scholar] [CrossRef]
- Gheonea, I.; Aprodu, I.; Râpeanu, G.; Stănciuc, N. Binding mechanisms between lycopene extracted from tomato peels and bovine β-lactoglobulin. J. Lumin. 2018, 203, 582–589. [Google Scholar]
- Kaur, M.; Bawa, M.; Singh, M. β-Carotene-β-cyclodextrin inclusion complex: Towards enhanced aqueous solubility. J. Glob. Biosci. 2016, 5, 3665–3675. [Google Scholar]
- Durante, M.; Milano, F.; De Caroli, M.; Giotta, L.; Piro, G.; Mita, G.; Frigione, M.; Lenucci, M.S. Tomato oil encapsulation by α-, β-, and γ-Cyclodextrins: A comparative study on the formation of supramolecular structures, antioxidant activity, and carotenoid stability. Foods 2020, 9, 1553. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, M.; Tavakoli, O.; Khoobi, M.; Wu, Y.S.; Faramarzi, M.A.; Gholibegloo, E.; Farkhondeh, S. Beta-carotene/cyclodextrin-based inclusion complex: Improved loading, solubility, stability, and cytotoxicity. J. Incl. Phenom. Macrocycl. Chem. 2022, 102, 55–64. [Google Scholar] [CrossRef]
- Lakshmanan, M.; Moses, J.A.; Chinnaswamy, A. Encapsulation of β-carotene in 2-hydroxypropyl-β-cyclodextrin/carrageenan/soy protein using a modified spray drying process. Int. J. Food Sci. Technol. 2022, 57, 2680–2688. [Google Scholar] [CrossRef]
- Nalawade, P.; Gajjar, A. Assessment of in-vitro bio accessibility and characterization of spray dried complex of astaxanthin with methylated betacyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2015, 83, 63–75. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Seo, T.-R.; Lim, S.-T. Preparation of aqueous dispersion of β-carotene nano-composites through complex formation with starch dextrin. Food Hydrocoll. 2013, 33, 256–263. [Google Scholar] [CrossRef]
- Kong, L.; Bhosale, R.; Ziegler, G.R. Encapsulation and stabilization of β-carotene by amylose inclusion complexes. Food Res. Int. 2018, 105, 446–452. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Huber, K.C. Preparation and characterization of corn starch-β-carotene composites. Carbohydr. Polym. 2016, 136, 394–401. [Google Scholar] [CrossRef]
- Allahdad, Z.; Khammari, A.; Karami, L.; Ghasemi, A.; Sirotkin, V.A.; Haertlé, T.; Saboury, A.A. Binding studies of crocin to β-Lactoglobulin and its impacts on both components. Food Hydrocoll. 2020, 108, 106003. [Google Scholar] [CrossRef]
- Buszewski, B.; Rodzik, A.; Railean-Plugaru, V.; Sprynskyy, M.; Pomastowski, P. A study of zinc ions immobilization by β-lactoglobulin. Colloids. Surf. A. Physicochem. Eng. Asp. 2020, 591, 124443. [Google Scholar] [CrossRef]
- Wang, C.; Chen, L.; Lu, Y.; Liu, J.; Zhao, R.; Sun, Y.; Sun, B.; Cuina, W. pH-Dependent complexation between β-lactoglobulin and lycopene: Multi-spectroscopy, molecular docking and dynamic simulation study. Food Chem. 2021, 362, 130230. [Google Scholar] [CrossRef]
- Thakur, S.; Sharma, R.; Garg, T. A review on microparticles: Preparation techniques and evaluation. Pharma Innov. J. 2022, 11, 837–840. [Google Scholar]
- Zhu, Z. Effects of amphiphilic diblock copolymer on drug nanoparticle formation and stability. Biomaterials 2013, 34, 10238–10248. [Google Scholar] [CrossRef] [PubMed]
- Cordt, C.; Meckel, T.; Geissler, A.; Biesalski, M. Entrapment of hydrophobic biocides into cellulose acetate nanoparticles by nanoprecipitation. Nanomaterials 2020, 10, 2447. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-Y.; Hsieh, Y.-S.; Wu, Y.-T. Poly (Lactic-Co-Glycolic) Acid–Poly (Vinyl Pyrrolidone) Hybrid Nanoparticles to Improve the Efficiency of Oral Delivery of β-Carotene. Pharmaceutics 2022, 14, 637. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Liu, W.; Yan, L.; Kong, F.; Wei, K. Optimization and characterization of poly (lactic-co-glycolic acid) nanoparticles loaded with astaxanthin and evaluation of anti-photodamage effect in vitro. R. Soc. Open Sci. 2019, 6, 191184. [Google Scholar] [CrossRef]
- Nalawade, P.; Gajjar, A. Optimization of astaxanthin microencapsulation in hydrophilic carriers using response surface methodology. Arch. Pharm. Res. 2015, 1–17. [Google Scholar] [CrossRef]
- Zhao, C.; Cheng, H.; Jiang, P.; Yao, Y.; Han, J. Preparation of lutein-loaded particles for improving solubility and stability by Polyvinylpyrrolidone (PVP) as an emulsion-stabilizer. Food Chem. 2014, 156, 123–128. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, H.; Liu, C.; Zhang, S.; Xu, Y.; Wang, D. Fabrication and characterization of lutein-loaded nanoparticles based on zein and sophorolipid: Enhancement of water solubility, stability, and bioaccessibility. J. Agric. Food Chem. 2019, 67, 11977–11985. [Google Scholar] [CrossRef]
- Suzuki, R.; Yasuhara, K.; Deguchi, S. Effect of Molecular Distortion on the Optical Properties of Carotenoid-Based Nanoparticles. J. Phys. Chem. C 2022, 126, 2607–2613. [Google Scholar] [CrossRef]
- Anarjan, N.; Tan, C.P.; Nehdi, I.A.; Ling, T.C. Colloidal astaxanthin: Preparation, characterisation and bioavailability evaluation. Food Chem. 2012, 135, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Crystals and crystallization in oil-in-water emulsions: Implications for emulsion-based delivery systems. Adv. Colloid Interface Sci. 2012, 174, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured lipid carriers: A groundbreaking approach for transdermal drug delivery. Adv. Pharm. Bull. 2020, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Doktorovova, S.; Shegokar, R.; Souto, E.B. Role of excipients in formulation development and biocompatibility of lipid nanoparticles (SLNs/NLCs). In Nanostructures for Novel Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 811–843. [Google Scholar]
- Ribeiro, A.P.B.; Masuchi, M.H.; Miyasaki, E.K.; Domingues, M.A.F.; Stroppa, V.L.Z.; de Oliveira, G.M.; Kieckbusch, T.G. Crystallization modifiers in lipid systems. J. Food Sci. Technol. 2015, 52, 3925–3946. [Google Scholar] [CrossRef]
- Weiss, J.; Decker, E.A.; McClements, D.J.; Kristbergsson, K.; Helgason, T.; Awad, T. Solid lipid nanoparticles as delivery systems for bioactive food components. Food Biophys. 2008, 3, 146–154. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Li, Y.; Kaul, N.; Abbaspourrad, A. Study of the physicochemical properties of fish oil solid lipid nanoparticle in the presence of palmitic acid and quercetin. J. Agric. Food Chem. 2019, 67, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Helgason, T.; Awad, T.S.; Kristbergsson, K.; Decker, E.A.; McClements, D.J.; Weiss, J. Impact of surfactant properties on oxidative stability of β-carotene encapsulated within solid lipid nanoparticles. J. Agric. Food Chem. 2009, 57, 8033–8040. [Google Scholar] [CrossRef]
- Gomes, G.V.d.L.; Borrin, T.R.; Cardoso, L.P.; Souto, E.; Pinho, S.C.d. Characterization and shelf life of β-carotene loaded solid lipid microparticles produced with stearic acid and sunflower oil. Braz Arch Biol Technol. 2013, 56, 663–671. [Google Scholar] [CrossRef]
- Chen, Y.; He, N.; Yang, T.; Cai, S.; Zhang, Y.; Lin, J.; Huang, M.; Chen, W.; Zhang, Y.; Hong, Z. Fucoxanthin Loaded in Palm Stearin-and Cholesterol-Based Solid Lipid Nanoparticle-Microcapsules, with Improved Stability and Bioavailability In Vivo. Mar. Drugs 2022, 20, 237. [Google Scholar] [CrossRef]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Design and characterization of astaxanthin-loaded nanostructured lipid carriers. Innov. Food Sci. Emerg. Technol. 2014, 26, 366–374. [Google Scholar] [CrossRef]
- Osanlou, R.; Emtyazjoo, M.; Banaei, A.; Hesarinejad, M.A.; Ashrafi, F. Preparation of solid lipid nanoparticles and nanostructured lipid carriers containing zeaxanthin and evaluation of physicochemical properties. Colloids. Surf., A. Physicochem. Eng. Asp. 2022, 641, 128588. [Google Scholar] [CrossRef]
- Xia, Z.; McClements, D.J.; Xiao, H. Influence of physical state of β-carotene (crystallized versus solubilized) on bioaccessibility. J. Agric. Food Chem. 2015, 63, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Y.; Mao, Y.; Zhao, Y.; Li, X.; Hu, J.; Li, Y. Effects of mono-and di-glycerides/phospholipids (MDG/PL) on the bioaccessibility of lipophilic nutrients in a protein-based emulsion system. Food Funct. 2022, 13, 8168–8178. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Cheng, G.; Gu, J.-C.; Xu, C.-H. Development of solid self-emulsifying drug delivery systems: Preparation techniques and dosage forms. Drug Discov. Today 2008, 13, 606–612. [Google Scholar] [CrossRef]
- Kim, M.-S.; Ha, E.-S.; Choo, G.-H.; Baek, I.-H. Preparation and in vivo evaluation of a dutasteride-loaded solid-supersaturatable self-microemulsifying drug delivery system. Int. J. Mol. Sci. 2015, 16, 10821–10833. [Google Scholar] [CrossRef]
- Dokania, S.; Joshi, A.K. Self-microemulsifying drug delivery system (SMEDDS)–challenges and road ahead. Drug Deliv. 2015, 22, 675–690. [Google Scholar] [CrossRef]
- Quan, G.; Niu, B.; Singh, V.; Zhou, Y.; Wu, C.-Y.; Pan, X.; Wu, C. Supersaturable solid self-microemulsifying drug delivery system: Precipitation inhibition and bioavailability enhancement. Int. J. Nanomed. 2017, 12, 8801. [Google Scholar] [CrossRef] [Green Version]
- Faisal, W.; Ruane-O’Hora, T.; O’Driscoll, C.M.; Griffin, B.T. A novel lipid-based solid dispersion for enhancing oral bioavailability of Lycopene–In vivo evaluation using a pig model. Int. J. Pharm. 2013, 453, 307–314. [Google Scholar] [CrossRef]
- Aung, W.T.; Khine, H.E.E.; Chaotham, C.; Boonkanokwong, V. Production, physicochemical investigations, antioxidant effect, and cellular uptake in Caco-2 cells of the supersaturable astaxanthin self-microemulsifying tablets. Eur. J. Pharm. Sci. 2022, 176, 106263. [Google Scholar] [CrossRef]
- Li, T.; Cipolla, D.; Rades, T.; Boyd, B.J. Drug nanocrystallisation within liposomes. J. Control. Release 2018, 288, 96–110. [Google Scholar] [CrossRef]
- Pan, L.; Wang, H.; Gu, K. Nanoliposomes as vehicles for astaxanthin: Characterization, in vitro release evaluation and structure. Molecules 2018, 23, 2822. [Google Scholar] [CrossRef] [PubMed] [Green Version]














| Carotenoids | Formulation | Composition | Crystalline Status | Results | Reference |
|---|---|---|---|---|---|
| Carotenoids | Co-crystal | Sucrose | Crystals | Thermal stability↑ | [70] |
| β-carotene | Solid dispersion | Poly (vinyl pyrrolidone) Sucrose fatty acid ester (S-1670) | Amorphs | Solubility↑ Dissolution↑ Bioavailability↑ | [75,76,77] |
| β-carotene | Solid dispersion | Cyclic amylopection | Amorphs | Stability↑ | [78] |
| β-carotγene | Inclusion complex | Amylose (Amylomaize starch) | Both Amorphs and crystals | Stability↑ | [89] |
| β-carotene | Inclusion complex | Amylose (Corn starch) | Both Amorphs and crystals | Stability↑ | [91] |
| β-carotene | Inclusion complex | Amylose (High-amylose corn starch) | Amorphs | Stability↑ | [90] |
| β-carotene | Inclusion complex | 2-hydroxylproply-β-cyclodextrin Carrageenan Soy protein | Amorphs | Bioaccessibility↑ | [87] |
| β-carotene | Nanoparticles | Poly (lactic-co-glycolic) acid Poly (vinyl pyrrolidone) | Amorphs | Bioavailability↑ | [98] |
| β-carotene | Nanoemulsion | Corn oil | Amorphs | Bioaccessibility↑ | [117] |
| β-carotene | Solid lipid microparticles | Stearic acid Sunflower oil | Amorphs or Less ordered crystals | Stability↑ | [113] |
| Lycopene | Solid dispersion (Dripping pills) | PEG 6000 Cremophor® EL Tween® 80 | Amorphs | Dissolution↑ Bioavailability↑ | [79] |
| Lycopene (Tomato oil) | Inclusion complex | α, β, γ-cyclodextrin | Microcrystals | Color change Stability↑ Antioxidation↑ | [85] |
| Lycopene | Lipid based solid dispersion | Gelucire 44/14 | Polymorphs | Dissolution↑ Bioavailability↑ | [123] |
| Astaxanthin | Inclusion complex | Methyl-β-cyclodextrin | Amorphs | Solubility↑ Dissolution↑ Bioaccessibility↑ | [88] |
| Astaxanthin | Colloidal particles | Tween® 20 Sodium caseinate Gum arabic | Polymorphs | Dissolution↑ Cellular uptake↑ | [104] |
| Astaxanthin | Microparticles | Povidone K30 Copovidone PEG 6000 Poloxamer 188 Tocopherol Colloidal silicon dioxide | Amorphs | HepG2 cell growth inhibition activity↑ | [100] |
| Astaxanthin | Nanoparticles | Poly(lactic-co-glycolic acid) | Amorphs | Cellular uptake↑ Photoprotection↑ | [99] |
| Astaxanthin | Liposome | Soybean phosphatidyl choline Cholesterol | Both Amorphs and crystals | Solubility↑ Stability↑ | [126] |
| Astaxanthin | Nanostructured lipid carrier | Glyceryl behenate Oleic acid Lecithin Tween® 80 | Amorphs | Stability↑ | [115] |
| Astaxanthin | Self-microemulsifying drug delivery system | Rice bran oil Kolliphor® RH 40 Span® 20 HPMC Polyvinyl alcohol | Amorphs | Dissolution↑ Antioxidation↑ Cellular uptake↑ | [124] |
| Fucoxanthin | Inclusion complex | 2-hydroxylpropyl-β-cyclodextrin | Amorphs | Solubility↑ Stability↑ Anti-tumor activity↑ | [81] |
| Fucoxanthin | Solid lipid nanoparticle-microcapsules | Palm stearin Cholesterol | Amorphs | Solubility↑ Stability↑ Bioavailability↑ | [114] |
| Lutein | Particles | Polyvinylpyrrolidone Tween® 80 | Amorphs | Stability↑ | [101] |
| Lutein | Nanoparticles | Zein Sophorolipid | Amorphs | Solubility↑ Bioaccessibility↑ | [102] |
| Lutein | Nanoemulsion | Blending plant oil Mono- and di-glycerides Lecithin Whey protein | Amorphs | Dissolution↑ Stability↑ | [118] |
| Zeaxanthin | Solid lipid nanoparticles | Glycerol monostearate Glycerol distearate | Possible amorphs | Dissolution↑ | [116] |
| Zeaxanthin | Nanostructured lipid carrier | Glycerol monostearate Glycerol distearate Medium-chain triglyceride Soy lecithin Tween® 80 | Possible amorphs | Dissolution↑ | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.-Y.; Hsieh, Y.-S.; Ko, H.-H.; Wu, Y.-T. Formulation Approaches to Crystalline Status Modification for Carotenoids: Impacts on Dissolution, Stability, Bioavailability, and Bioactivities. Pharmaceutics 2023, 15, 485. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics15020485
Liu W-Y, Hsieh Y-S, Ko H-H, Wu Y-T. Formulation Approaches to Crystalline Status Modification for Carotenoids: Impacts on Dissolution, Stability, Bioavailability, and Bioactivities. Pharmaceutics. 2023; 15(2):485. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics15020485
Chicago/Turabian StyleLiu, Wan-Yi, Yun-Shan Hsieh, Horng-Huey Ko, and Yu-Tse Wu. 2023. "Formulation Approaches to Crystalline Status Modification for Carotenoids: Impacts on Dissolution, Stability, Bioavailability, and Bioactivities" Pharmaceutics 15, no. 2: 485. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics15020485