Enhancing Pharmaceutical Packaging through a Technology Ecosystem to Facilitate the Reuse of Medicines and Reduce Medicinal Waste
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Technologies for Quality Requirements
3.2. Technologies for Safety Requirements
4. Discussion
- (i)
- Thin-film technologiesPrinted electronics and nanotechnology mentioned previously provide methods to place electronic circuits on packaging materials. However, these technologies are still not common and complicated circuitry such as wireless modules and high-power microprocessors are still not directly printable onto the packaging surface.
- (ii)
- Energy harvestingRFID is normally used to provide power to read a passive tag but a continuous power supply for maintaining the regular sensing and the network connection is required. Technology for printed batteries is still in an early stage [108], energy harvesting techniques such as extracting ambient energy could be an alternative [109], and wireless charging can also be a good candidate supplying continuous power to the embedded electronics from a distance [110]. However, all these technologies are not yet mature enough for immediate implementation onto intelligent pharmaceutical packaging.
- (iii)
- Flexible displayFlexible displays using e-ink or EC technology show a promising way to use minimum energy to sustain a dynamic changing electronic display mounted on existing flat or curved pharmaceutical packaging. Although no power is required for maintaining e-ink screen contents, the irregular updates still require a significant amount of electrical power to align the color pigments. Electrochromism technology reduces the energy for updating EC displays but a regular refresh process is required to keep the screen content visible. New low cost, low energy and printable technologies for pharmaceutical packaging are required.
- (a)
- patients’ incentive for returning unwanted medicines,
- (b)
- pharmacists’ incentive for extra workload in re-dispensing medicines,
- (c)
- cost effectiveness monitoring of reusing medicines,
- (d)
- legal issues such as legislation on re-dispensing medicines and professional standards for pharmacists,
- (e)
- social norm for promoting medicine reuse,
- (f)
- on-site and off-site collection and distribution system.
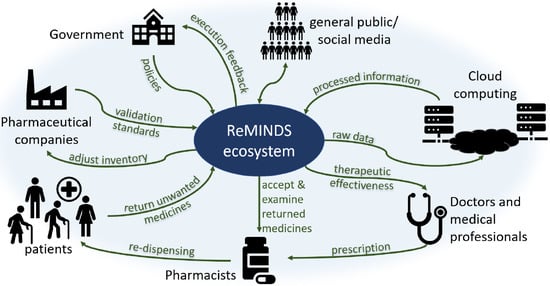
5. ReMINDS Ecosystem
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hazell, B.; Robson, R. Pharmaceutical waste reduction in the NHS. Rep. Version 2015, 1, 1–24. [Google Scholar]
- Opar, A. Rising drug costs prompt new uses for old pills. Nat. Med. 2006, 12, 1333. [Google Scholar] [CrossRef] [PubMed]
- Toh, M.R.; Chew, L. Turning waste medicines to cost savings: A pilot study on the feasibility of medication recycling as a solution to drug wastage. Palliat. Med. 2017, 31, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Bekker, C.L.; Gardarsdottir, H.; Egberts, A.C.G.; Molenaar, H.A.; Bouvy, M.L.; van den Bemt, B.J.F.; Hövels, A.M. What does it cost to redispense unused medications in the pharmacy? A micro-costing study. BMC Health Serv. Res. 2019, 19, 243. [Google Scholar] [CrossRef]
- Trueman, P.; Taylor, D.; Lowson, K.; Bligh, A.; Meszaros, A.; Wright, D.; Glanville, J.; Newbould, J.; Bury, M.; Barber, N.; et al. Evaluation of the Scale, Causes and Costs of Waste Medicines. Report of DH Funded National Project; York Health Economics Consortium and The School of Pharmacy, University of London: London, UK, 2010. [Google Scholar]
- Bekker, C.; Gardarsdottir, H.; Egberts, A.; Bouvy, M.; van den Bemt, B. Worldwide initiatives to decrease medication wastage: A cross-national survey. In Proceedings of the 44th ESCP International Symposium on Clinical Pharmacy Medicines Information: Making Better Decisions, Lisbon, Portugal, 30 October 2015; Volume 38, p. 591. [Google Scholar]
- Connelly, D. Should pharmacists be allowed to reuse medicines. Pharm. J. 2019. [Google Scholar] [CrossRef]
- Kaldy, J. Program Turns Discarded Drugs Into Lifesavers for Needy. Caring Ages 2015, 16, 9. [Google Scholar] [CrossRef]
- Cauchi, R.; Berg, K. State Prescription Drug Return, Reuse and Recycling Laws. Available online: http://www.ncsl.org/research/health/state-prescription-drug-return-reuse-and-recycling.aspx (accessed on 10 November 2019).
- Crews, J. Prescription Drug Reuse and Recycling; Taylor & Francis: Abingdon, UK, 2019. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drug Donations; World Health Organization: Geneva, Switzerlnad, 1999; Volume 20. [Google Scholar]
- Gotink, M.H.; Ralitapole, D.K. Inter Care: Help for rural African hospitals. Br. Med J. 1988, 297, 1402. [Google Scholar] [CrossRef] [Green Version]
- Viegas, C.V.; Bond, A.; Vaz, C.R.; Bertolo, R.J. Reverse flows within the pharmaceutical supply chain: A classificatory review from the perspective of end-of-use and end-of-life medicines. J. Clean. Prod. 2019, 238, 117719. [Google Scholar] [CrossRef]
- Trappey, A.J.C.; Trappey, C.V.; Fan, C.Y.; Hsu, A.P.T.; Li, X.K.; Lee, I.J.Y. IoT patent roadmap for smart logistic service provision in the context of Industry 4.0. J. Chin. Inst. Eng. 2017, 40, 593–602. [Google Scholar] [CrossRef]
- Ding, B. Pharma Industry 4.0: Literature review and research opportunities in sustainable pharmaceutical supply chains. Process. Saf. Environ. Prot. 2018, 119, 115–130. [Google Scholar] [CrossRef]
- Bekker, C.L.; Gardarsdottir, H.; Egberts, T.C.G.; Bouvy, M.L.; van den Bemt, B.J.F. Redispensing of medicines unused by patients: A qualitative study among stakeholders. Int. J. Clin. Pharm. 2017, 39, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Bekker, C.; van den Bemt, B.; Egberts, T.C.G.; Bouvy, M.; Gardarsdottir, H. Willingness of patients to use unused medication returned to the pharmacy by another patient: A cross-sectional survey. BMJ Open 2019, 9, 1–5. [Google Scholar] [CrossRef] [PubMed]
- McRae, D.; Allman, M.; James, D. The redistribution of medicines: Could it become a reality? Int. J. Pharm. Pract. 2016, 24, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Alhamad, H.; Patel, N.; Donyai, P. How do people conceptualise the reuse of medicines? An interview study. Int. J. Pharm. Pract. 2018, 26, 232–241. [Google Scholar] [CrossRef]
- Black, G. Reuse of medicine: It’s not about the money!: From my Little Black Book of pharmacy practice: Practice matters. SA Pharm. J. 2011, 78, 51–53. [Google Scholar]
- Lorenzini, G.C.; Mostaghel, R.; Hellstrom, D. Drivers of pharmaceutical packaging innovation: A customer-supplier relationship case study. J. Bus. Res. 2018, 88, 363–370. [Google Scholar] [CrossRef]
- Munzel, J. Pharmaceutical packaging: Technology and design requirements are on the rise. J. Med Mark. 2007, 7, 136–145. [Google Scholar] [CrossRef]
- Zadbuke, N.; Shahi, S.; Gulecha, B.; Padalkar, A.; Thube, M. Recent trends and future of pharmaceutical packaging technology. J. Pharm. Bioallied Sci. 2013, 5, 98–110. [Google Scholar] [CrossRef]
- Sehgal, S.; Jaithliya, T.; Khan, M.; Devi, A.N.; Banoo, J.; Tiwari, A. Recent trends and future of pharmaceutical packaging technology: An overview. Eur. J. Biomed. Pharm. Sci. 2018, 5, 957–966. [Google Scholar]
- Madhusudan, P.; Chellukuri, N.; Shivakumar, N. Smart packaging of food for the 21st century—A review with futuristic trends, their feasibility and economics. Mater. Today Proc. 2018, 5, 21018–21022. [Google Scholar] [CrossRef]
- Hara, L.; Guirguis, R.; Hummel, K.; Villanueva, M. More Than Bar Codes: Integrating Global Standards-Based Bar Code Technology Into National Health Information Systems in Ethiopia and Pakistan to Increase End-to-End Supply Chain Visibility. Glob. Health Sci. Pract. 2017, 5, 678–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavorgna, A. The online trade in counterfeit pharmaceuticals: New criminal opportunities, trends and challenges. Eur. J. Criminol. 2015, 12, 226–241. [Google Scholar] [CrossRef]
- Degardin, K.; Guillemain, A.; Klespe, P.; Hindelang, F.; Zurbach, R.; Roggo, Y. Packaging analysis of counterfeit medicines. Forensic Sci. Int. 2018, 291, 144–157. [Google Scholar] [CrossRef]
- Patel, R.P.; Patel, Y.B.; Prajapati, B.G.; Borkhataria, C.H. Outline of Pharmaceutical Packaging Technology. Int. Res. J. Pharm. 2010, 1, 105–112. [Google Scholar]
- Janjarasskul, T.; Suppakul, P. Active and intelligent packaging: The indication of quality and safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 808–831. [Google Scholar] [CrossRef]
- Selman, J.D. Time-temperature indicators. In Active Food Packaging; Springer US: Boston, MA, USA, 1995; pp. 215–237. [Google Scholar] [CrossRef]
- Usak, M.; Kubiatko, M.; Shabbir, M.S.; Viktorovna Dudnik, O.; Jermsittiparsert, K.; Rajabion, L. Health care service delivery based on the Internet of things: A systematic and comprehensive study. Int. J. Commun. Syst. 2020, 33, e4179. [Google Scholar] [CrossRef]
- Müller, P.; Schmid, M. Intelligent packaging in the food sector: A brief overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Kleppe, M.; Lacroix, J.; Ham, J.; Midden, C. A dual-process view on medication adherence: The role of affect. J. Health Psychol. 2017, 24, 1033–1042. [Google Scholar] [CrossRef]
- Martin, L.R.; Feig, C.; Maksoudian, C.R.; Wysong, K.; Faasse, K. A perspective on nonadherence to drug therapy: Psychological barriers and strategies to overcome nonadherence. Patient Prefer. Adherence 2018, 12, 1527–1535. [Google Scholar] [CrossRef] [Green Version]
- Swartz, M.K. The PRISMA Statement: A Guideline for Systematic Reviews and Meta-Analyses. J. Pediatr. Health Care 2011, 25, 1–2. [Google Scholar] [CrossRef]
- Waffenschmidt, S.; Knelangen, M.; Sieben, W.; Bühn, S.; Pieper, D. Single screening versus conventional double screening for study selection in systematic reviews: A methodological systematic review. BMC Med. Res. Methodol. 2019, 19, 132. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Hubley, J. Understanding Behaviour: The Key to Successful Health Education. Trop. Dr. 1988, 18, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, H.; Su, H.M.; Imani, S.M.; Alkhaldi, K.M.; Filipe, C.D.; Didar, T.F. Intelligent Food Packaging: A Review of Smart Sensing Technologies for Monitoring Food Quality. ACS Sensors 2019, 4, 808–821. [Google Scholar] [CrossRef]
- Kuswandi, B.; Wicaksono, Y.; Jayus; Abdullah, A.; Heng, L.Y.; Ahmad, M. Smart Packaging: Sensors for monitoring of food quality and safety. Sens. Instrum. Food Qual. Saf. 2011, 5, 137–146. [Google Scholar] [CrossRef]
- Vanderroost, M.; Ragaert, P.; Devlieghere, F.; De Meulenaer, B. Intelligent food packaging: The next generation. Trends Food Sci. Technol. 2014, 39, 47–62. [Google Scholar] [CrossRef]
- Pavelková, A. Time temperature indicators as devices intelligent packaging. ACTA Univ. Agric. Silvic. Mendel. Brun. 2013, 61, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Liu, X.; Yang, M.; Zhang, Y.; Xiang, K.; Tang, R. Review of Time Temperature Indicators as Quality Monitors in Food Packaging. Packag. Technol. Sci. 2015, 28, 839–867. [Google Scholar] [CrossRef]
- Pereira, V.A., Jr.; de Arruda, I.N.Q.; Stefani, R. Active chitosan/PVA films with anthocyanins from Brassica oleraceae (Red Cabbage) as Time-Temperature Indicators for application in intelligent food packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar] [CrossRef]
- Mijanur Rahman, A.T.M.; Kim, D.H.; Jang, H.D.; Yang, J.H.; Lee, S.J. Preliminary study on biosensor-type time-temperature integrator for intelligent food packaging. Sensors 2018, 18, 1949. [Google Scholar] [CrossRef] [Green Version]
- Maslik, J.; Andersson, H.; Forsberg, V.; Engholm, M.; Zhang, R.; Olin, H. PEDOT: PSS temperature sensor ink-jet printed on paper substrate. J. Instrum. 2018, 13, C12010. [Google Scholar] [CrossRef]
- Maiellaro, G.; Ragonese, E.; Gwoziecki, R.; Jacobs, S.; Marjanović, N.; Chrapa, M.; Schleuniger, J.; Palmisano, G. Ambient light organic sensor in a printed complementary organic TFT technology on flexible plastic foil. IEEE Trans. Circuits Syst. Regul. Pap. 2013, 61, 1036–1043. [Google Scholar] [CrossRef]
- Feng, Y.; Xie, L.; Chen, Q.; Zheng, L. Low-Cost Printed Chipless RFID Humidity Sensor Tag for Intelligent Packaging. IEEE Sensors J. 2015, 15, 3201–3208. [Google Scholar] [CrossRef]
- Javed, N.; Habib, A.; Amin, Y.; Loo, J.; Akram, A.; Tenhunen, H. Directly Printable Moisture Sensor Tag for Intelligent Packaging. IEEE Sensors J. 2016, 16, 6147–6148. [Google Scholar] [CrossRef]
- Zhang, X.; Shan, X.; Wei, J. Hybrid flexible smart temperature tag with NFC technology for smart packaging. In Proceedings of the 19th Electronics Packaging Technology Conference, Singapore, 6 December 2017; pp. 1–5. [Google Scholar] [CrossRef]
- Falco, A.; Salmerón, J.F.; Loghin, F.C.; Lugli, P.; Rivadeneyra, A. Fully printed flexible single-chip RFID tag with light detection capabilities. Sensors 2017, 17, 534. [Google Scholar] [CrossRef]
- Kuswandi, B. Nanotechnology in Food Packaging. In Nanoscience in Food and Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; pp. 151–183. [Google Scholar] [CrossRef]
- Feng, Y.; Xie, L.; Mäntysalo, M.; Chen, Q.; Zheng, L. Electrical and humidity-sensing characterization of inkjetprinted multi-walled carbon nanotubes for smart packaging. Sensors IEEE 2013, 1–4. [Google Scholar] [CrossRef]
- Rahimi, R.; Zhou, J.; Jiang, H.; Soleimani, T.; Ziaia, B. Facile fabrication of low-cost passive wireless humidity sensor for smart packaging via all-laser processing of metalized paper. In Proceedings of the Solid-State Sensors, Actuators and Microsystems Workshop, Hilton Head Island, SC, USA; 2018; pp. 326–329. [Google Scholar] [CrossRef]
- Johnston, J.J.; Wong, J.P.; Feldman, S.E.; Ilnicki, L.P. Purge and Trap/Gas Chromatography/Mass Spectrometry Method for Determining Smoke Contamination of Foods and Packaging Materials. J. Agric. Food Chem. 1994, 42, 1954–1958. [Google Scholar] [CrossRef]
- Mielniczuk, Z.; Pogorzelska, Z. Detection of microbial contamination of packaging for foodstuffs by gas chromatography-mass spectrometry method. Packag. Technol. Sci. 2002, 15, 47–51. [Google Scholar] [CrossRef]
- Tretola, M.; Di Rosa, A.R.; Tirloni, E.; Ottoboni, M.; Giromini, C.; Leone, F.; Bernardi, C.E.M.; Dell’Orto, V.; Chiofalo, V.; Pinotti, L. Former food products safety: Microbiological quality and computer vision evaluation of packaging remnants contamination. Food Addit. Contam. Part A 2017, 34, 1427–1435. [Google Scholar] [CrossRef]
- Troja, D.; Shabani, L.; Troja, R. Packaging systems influcnce on the microbial contamination of common pharmaceutical products. J. Hyg. Eng. Des. 2014, 6, 142–146. [Google Scholar]
- White, K.; Lin, L.; Dahl, D.W.; Ritchie, R.J.B. When do consumers avoid imperfections? Superficial packaging damage as a contamination cue. J. Mark. Res. 2016, 53, 110–123. [Google Scholar] [CrossRef]
- Akbarian, M.; Ghasemi, Y.; Uversky, V.N.; Yousefi, R. Chemical modifications of insulin: Finding a compromise between stability and pharmaceutical performance. Int. J. Pharm. 2018, 547, 450–468. [Google Scholar] [CrossRef] [PubMed]
- Hii, M.S.Y.; Courtney, P.; Royall, P.G. An Evaluation of the Delivery of Medicines Using Drones. Drones 2019, 3, 52. [Google Scholar] [CrossRef] [Green Version]
- Jing, Q.; Xie, Y.; Zhu, G.; Han, R.P.S.; Wang, Z.L. Self-powered thin-film motion vector sensor. Nat. Commun. 2015, 6, 8031. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Harada, S.; Yamamoto, D.; Honda, W.; Arie, T.; Akita, S.; Takei, K. Printed multifunctional flexible device with an integrated motion sensor for health care monitoring. Sci. Adv. 2016, 2, e1601473. [Google Scholar] [CrossRef] [Green Version]
- Gong, L.; Yu, M.; Duan, W.; Ye, X.; Gudmundsson, K.; Swainson, M. A Novel Camera Based Approach for Automatic Expiry Date Detection and Recognition on Food Packages. In Artificial Intelligence Applications and Innovation. IFIP Advances in Information and Communication Technology; Springer: Cham, Switzerland, 2018; pp. 133–142. [Google Scholar] [CrossRef] [Green Version]
- Peng, E.; Peursum, P.; Li, L. Product Barcode and Expiry Date Detection for the Visually Impaired Using a Smartphone. In Proceedings of the International Conference on Digital Image Computing Techniques and Applications (DICTA), Perth, Australia, 3 Decemebr 2012; pp. 1–7. [Google Scholar] [CrossRef]
- Ramalingam, M.; Puviarasi, R.; Zakaria, N.D.A.B. Developing mobile application for medicine expiry date detection. Int. J. Pure Appl. Math. 2018, 119, 3895–3900. [Google Scholar]
- Blankenbach, K.; Duchemin, P.; Rist, B.; Bogner, D.; Krause, M. Smart Pharmaceutical Packaging with E-Paper Display for improved Patient Compliance. In SID Symposium Digest of Technical Papers; Wiley: Hoboken, NJ, USA, 2018; Volume 49, pp. 271–274. [Google Scholar]
- Kai, H.; Suda, W.; Ogawa, Y.; Nagamine, K.; Nishizawa, M. Intrinsically Stretchable Electrochromic Display by a Composite Film of Poly(3,4-ethylenedioxythiophene) and Polyurethane. ACS Appl. Mater. Interfaces 2017, 9, 19513–19518. [Google Scholar] [CrossRef]
- Brooke, R.; Edberg, J.; Crispin, X.; Berggren, M.; Engquist, I.; Jonsson, P.M. Greyscale and Paper Electrochromic Polymer Displays by UV Patterning. Polymers 2019, 11, 267. [Google Scholar] [CrossRef] [Green Version]
- Kumbhar, M.S.; Choudhary, N.H.; Dighe, D.A.; Singh, M.C. Tamper Evident Pharmaceutical Packaging - Needs and Advances. Int. J. Pharm. Sci. Rev. Res. 2012, 13, 141–153. [Google Scholar]
- CPG. Compliance Policy Guide Sec. 450.500 Tamper-Resistant Packaging Requirements for Certain Over-the-Counter Human Drug Products MAY 1992; Federal Register; US Food and Drug Administration: Washington, DC, USA, 2015; Volume 63, pp. 59463–59471.
- Kumar, A.K.; Gupta, N.V.; Lalasa, P.; Sandhil, S. A review on packaging materials with anti-counterfeit, tamper-evident features for pharmaceuticals. Int. J. Drug Dev. Res. 2013, 5, 26–34. [Google Scholar]
- O’Connor, M.C. Packaging Maker Offering Tamper-Evident RFID Film. RFID J. Available online: https://www.rfidjournal.com/articles/view?2959 (accessed on 24 March 2020).
- Jo, C.L.; Ambs, A.; Dresler, C.M.; Backinger, C.L. Child-resistant and tamper-resistant packaging: A systematic review to inform tobacco packaging regulation. Prev. Med. 2017, 95, 89–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naitove, M. In food packaging, peelable IML serves as tamper-evident seal. Plast. Technol. 2018, 64, 14–16. [Google Scholar]
- Mayberry, J. Make your mark: Tamper-evident packaging remains invaluable for safety-conscious consumers. Pharm. Process. 2013, 28, 36–37. [Google Scholar]
- Floerkemeier, C.; Siegemund, F. Improving the effectiveness of medical treatment with pervasive computing technologies. In Proceedings of the UbiHealth: The 2nd International Workshop on Ubiquitous Computing for Pervasive Healthcare Applications, Seattle, WA, USA, 12 October 2003. [Google Scholar]
- Gao, J.; Pang, Z.; Chen, Q.; Zheng, L.R. Interactive packaging solutions based on RFID technology and controlled delamination material. In Proceedings of the RFID 2010: International IEEE Conference on RFID, Orlando, FL, USA, 16 April 2010; pp. 158–165. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.B.; Rogers, J.; Jones, E.C. The impact of a shared pharmaceutical supply chain model on counterfeit drugs, diverted drugs, and drug shortages. In Proceedings of the Portland International Conference on Management of Engineering and Technology (PICMET), Portland, OR, USA, 2–6 August 2015; pp. 1879–1889. [Google Scholar] [CrossRef]
- Shah, R.Y.; Prajapati, P.N.; Agrawal, Y.K. Anticounterfeit packaging technologies. J. Adv. Pharm. Technol. Res. 2010, 1, 368–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, D.; Malla, S.; Gudala, K.; Tiwari, P. Anti-Counterfeit Technologies: A Pharmaceutical Industry Perspective. Sci. Pharm. 2013, 81, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Andria, S.E.; Fulcher, M.; Witkowski, M.R.; Platek, S.F. The Use of SEM/EDS and FT-IR Analyses in the Identification of Counterfeit Pharmaceutical Packaging. Am. Pharm. Rev. 2012, 15, 62. [Google Scholar]
- Li, Y.; Li, J. The Intelligent Texture Anti-counterfeiting Algorithm Based on DFT. In Proceedings of the Ninth International Conference on Intelligent Information Hiding and Multimedia Signal Processing, Beijing, China, 16 October 2013; pp. 590–593. [Google Scholar] [CrossRef]
- Simske, S.; Mucher, P.; Martinez, C. Using variable data security printing to provide customized package protection. Int. Conf. Digit. Prod. Print. Ind. Appl. Final. Program Proc. 2005, 3, 112–113. [Google Scholar]
- Ishiyama, R.; Takahashi, T.; Makino, K.; Kudo, Y.; Kooper, M.; Abbink, D. Medicine Tablet Authentication Using “Fingerprints” of Ink-Jet Printed Characters. In Proceedings of the International Conference on Industrial Technology (ICIT), Melbourne, Australia, 15 February 2019; pp. 871–876. [Google Scholar] [CrossRef]
- Fei, J.; Liu, R. Drug-laden 3D biodegradable label using QR code for anti-counterfeiting of drugs. Mater. Sci. Eng. 2016, 63, 657–662. [Google Scholar] [CrossRef]
- Ludasi, K.; Jójárt-Laczkovich, O.; Sovány, T.; Hopp, B.; Smausz, T.; Regdon, G., Jr. Comparison of conventionally and naturally coloured coatings marked by laser technology for unique 2D coding of pharmaceuticals. Int. J. Pharm. 2019, 570, 118665. [Google Scholar] [CrossRef]
- Han, S.; Bae, H.J.; Kim, J.; Shin, S.; Choi, S.; Lee, S.H.; Kwon, S.; Park, W. Lithographically encoded polymer microtaggant using high-capacity and error-correctable QR code for anti-counterfeiting of drugs. Adv. Mater. 2012, 24, 5924–5929. [Google Scholar] [CrossRef]
- Platek, S.F.; Ranieri, N.; Batson, J. Applications of the FDA’s Counterfeit Detection Device (CD3+) to the Examination of Suspect Counterfeit Pharmaceutical Tablets and Packaging. Microsc. Microanal. 2016, 22. [Google Scholar] [CrossRef] [Green Version]
- Kwok, K.; Taylor, L.S. Analysis of the packaging enclosing a counterfeit pharmaceutical tablet using Raman microscopy and two-dimensional correlation spectroscopy. Vib. Spectrosc. 2012, 61, 176–182. [Google Scholar] [CrossRef]
- Eliasson, C.; Matousek, P. Noninvasive Authentication of Pharmaceutical Products through Packaging Using Spatially Offset Raman Spectroscopy. Anal. Chem. 2007, 79, 1696–1701. [Google Scholar] [CrossRef]
- Nilsson, E.; Nilsson, B.; Järpe, E. A pharmaceutical anti-counterfeiting method using time controlled numeric tokens. In Proceedings of the International Conference on RFID-Technologies and Applications, Sitges, Spain, 15 March 2011; pp. 343–347. [Google Scholar] [CrossRef] [Green Version]
- Al-Bahri, M.; Yankovsky, A.; Kirichek, R.; Borodin, A. Smart System Based on DOA & IoT for Products Monitoring & Anti-Counterfeiting. In Proceedings of the 4th MEC International Conference on Big Data and Smart City (ICBDSC), Muscat, Oman, 15–16 January 2019; pp. 1–5. [Google Scholar] [CrossRef]
- Garankina, R.Y.; Zakharochkina, E.R.; Samoshchenkova, I.F.; Lebedeva, N.Y.; Lebedev, A.V. Blockchain Technology and its Use in the Area of Circulation of Pharmaceuticals. J. Pharm. Sci. Res. 2018, 10, 2715–2717. [Google Scholar]
- Pashkov, V.; Soloviov, O. Legal implementation of blockchain technology in pharmacy. In Proceedings of the 7th International Interdisciplinary Scientific Conference: Society, Health, Welfare, Rīga Stradiņš University (RSU), Rīga, Latvia, 10–12 October 2018; Volume 68, pp. 1–8. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Yi, D.; Kuang, J. Pharmaceutical Supply Chain Management System with Integration of IoT and Blockchain Technology; Smart Blockchain; Qiu, M., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 97–108. [Google Scholar]
- Ogden, J. Implementing the EU Falsified Medicines Directive. Prescriber 2019, 30, 30–33. [Google Scholar] [CrossRef] [Green Version]
- Nowakowski, W. The Internet of Things: From smart packaging to a world of smart objects? Elektronika 2016, 10, 70–75. [Google Scholar] [CrossRef]
- Suganya, G.; Premalatha, M.; Anushka, S.; Muktak, P.; Abhishek, J. IoT based Automated Medicine Dispenser for Online Health Community using Cloud. Int. J. Recent Technol. Eng. 2019, 7, 1–4. [Google Scholar]
- Thakkar, H.; Trivedi, V.; Jolapara, U.; Chauhan, J. MED-IoT: A Medicine Confirmation System. In Proceedings of the International Conference on Smart City and Emerging Technology (ICSCET), Mumbai, India, 5 January 2018; pp. 1–5. [Google Scholar] [CrossRef]
- López-Nores, M.; Pazos-Arias, J.J.; García-Duque, J.; Blanco-Fernández, Y.; Ramos-Cabrer, M. Introducing smart packaging in residential networks to prevent medicine misuse. In Proceedings of the IEEE International Symposium on Consumer Electronics, Las Vegas, NV, USA, 9 January 2008; pp. 1–3. [Google Scholar] [CrossRef]
- Srinivas, M.; Durgaprasadarao, P.; Raj, V.N.P. Intelligent medicine box for medication management using IoT. In Proceedings of the 2nd International Conference on Inventive Systems and Control (ICISC), JCT College of Engineering and Technology, Tamil Nadu, India, 19–20 January 2018; pp. 32–34. [Google Scholar] [CrossRef]
- da Silva, D.V.; Gonçalves, T.G.; Pires, P.F. Using IoT technologies to develop a low-cost smart medicine box. In Proceedings of the 25th Brazillian Symposium on Multimedia and the Web, WebMedia 2019, Espírito Santo, Brazil, 26 October 2019; pp. 97–101. [Google Scholar] [CrossRef]
- Yang, G.; Xie, L.; Mantysalo, M.; Zhou, X.L.; Pang, Z.B.; Xu, L.D.; Kao-Walter, S.; Chen, Q.; Zheng, L.R. A Health-IoT Platform Based on the Integration of Intelligent Packaging, Unobtrusive Bio-Sensor, and Intelligent Medicine Box. IEEE Trans. Ind. Inform. 2014, 10, 2180–2191. [Google Scholar] [CrossRef] [Green Version]
- Pang, Z.; Tian, J.; Chen, Q. Intelligent packaging and intelligent medicine box for medication management towards the Internet-of-Things. In Proceedings of the 16th International Conference on Advanced Communication Technology, PyeongChang, Korea, 16 February 2014; Volume 2, pp. 352–360. [Google Scholar] [CrossRef]
- Farahani, B.; Firouzi, F.; Chang, V.; Badaroglu, M.; Constant, N.; Mankodiya, K. Towards fog-driven IoT eHealth: Promises and challenges of loT in medicine and healthcare. Future Gener. Comput. Syst. 2018, 78, 659–676. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Shin, J.; Yin, L.; You, J.M.; Meng, Y.S.; Wang, J. All-Printed, Stretchable Zn-Ag2O Rechargeable Battery via Hyperelastic Binder for Self-Powering Wearable Electronics. Adv. Energy Mater. 2017, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Garg, N.; Garg, R. Energy harvesting in IoT devices: A survey. In Proceedings of the International Conference on Intelligent Sustainable Systems (ICISS), SCAD Institute of Technology at Palladam, Tirupur, India, 7–8 December 2017; pp. 127–131. [Google Scholar] [CrossRef]
- Meile, L.; Ulrich, A.; Magno, M. Wireless Power Transmission Powering Miniaturized Low Power IoT devices: A Review. In Proceedings of the 8th International Workshop on Advances in Sensors and Interfaces (IWASI), Otranto, Italy, 14 June 2019; pp. 312–317. [Google Scholar] [CrossRef]


| Requirements | Quality | Safety | Others |
|---|---|---|---|
| Patients’ perspective [17] | (1) storage and handling conditions. | (1) tamper-proof packaging; (2) anti-counterfeit. | (1) patient incentive; (2) cost effectiveness. |
| Healthcare professionals’ perspective [18] | (1) storage conditions (temperature, moisture and light); (2) contamination of package (stain, smell); (3) last dispensing date. | (1) tamper-proof packaging; (2) anti-counterfeit. | (1) cost effectiveness; (2) legal issues regarding pharmacist responsibility, medicine recall, paperwork, efficacy, and governmental regulations. |
| Stakeholders’ perspective [16] | (1) monitor storage conditions (temperature, light, humidity, agitation, and lapsed expiration date). | (1) anti-counterfeit; (2) track and trace system to the packages for re-dispensed medicines. | (1) patients’ incentive; (2) pharmacists’ incentive; (3) cost benefits shared by stakeholders (patients, pharmacists and health insurance companies). |
| TPB Behavioral beliefs [19] | (1) storage conditions (temperature, humidity and cleanliness); (2) contaminated packaging. | (1) tamper-proof packaging; (2) errors introduced by patients or pharmacists; (3) anti-counterfeit. | (1) cost effectiveness. |
| TPB Normative beliefs [19] | Nil | Nil | (1) concern mostly on the social norm for reusing medicines. |
| TPB Control beliefs [19] | (1) monitor storage conditions (temperature, light, humidity, agitation, and lapsed expiration date). | (1) tamper-proof packaging; (2) anti-counterfeit. | (1) patient incentive; (2) on-site and off-site collection and distribution system. |
| Requirements | Technologies | Keywords for Search |
|---|---|---|
| Quality | (1) storage temperature monitoring (2) storage humidity monitoring (3) storage lighting monitoring (4) storage contamination monitoring (5) agitation monitoring (6) lapsed expiration date monitoring | (1) (intelligent OR smart OR monitor) AND packaging AND temperature (2) (intelligent OR smart OR monitor) AND packaging AND (humidity OR moisture) (3) (light OR optical OR UV) AND food AND packaging (4) packaging AND contamination (5) (vibration OR shock OR acceleration OR shake OR agitation) AND packaging (6) (report OR monitor OR detection) AND expiry |
| Safety | (1) tamper-proof packaging (2) anti-counterfeit (3) track & trace collecting and dispensing system (4) errors tracking from patients and pharmacists | (1) (evident OR resistant OR detection OR proof) AND tamper AND packaging (2–4) (pharmaceutical OR intelligent OR smart OR packaging) AND counterfeit |
| Requirements | Technologies |
|---|---|
| Quality | (i) storage temperature monitoring: passive TTI [43,44,45] (ii) storage temperature monitoring: active TTI with digital interfaces [46] (iii) thin-film technology: printed sensors and RFID tags [47,48,49,50] (iv) thin-film technology: hybrid printed circuits [51,52] (v) thin-film technology: nanotechnology [53,54,55] (vi) contamination detection: PT/GC/MS methodology [56,57] (vii) contamination detection: computer vision [58] (viii) contamination detection: tamper-evident check [59,60] (ix) motion detection: wearable sensors [63,64] (x) expiry date detection: visual inspection [66] (xi) expiry date detection: QR codes and smartphones [67] (xii) on packaging display: e-ink displays [68] and EC displays [69,70] |
| Safety | (i) tamper-proof: tamper-evident and tamper-resistance on packaging [71,72,73,75] (ii) tamper-proof: tamper-evident for transportation [74] (iii) tamper-proof: implementation during production [23,76] (iv) tamper-proof: built-in digital interfaces [77,78] (v) anti-counterfeit: overt/covert indications [80,81,82] (vi) anti-counterfeit: packaging materials inspection [83,84,85] (vii) anti-counterfeit: tagging on label, on medicine and on-dose [86,87,88,89] (viii) anti-counterfeit: readers for mini-size tags [90,91,92] (ix) anti-counterfeit: track and trace systems through Internet [93,94] (x) anti-counterfeit: open ledger based on blockchain [95,96,97] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hui, T.K.L.; Mohammed, B.; Donyai, P.; McCrindle, R.; Sherratt, R.S. Enhancing Pharmaceutical Packaging through a Technology Ecosystem to Facilitate the Reuse of Medicines and Reduce Medicinal Waste. Pharmacy 2020, 8, 58. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmacy8020058
Hui TKL, Mohammed B, Donyai P, McCrindle R, Sherratt RS. Enhancing Pharmaceutical Packaging through a Technology Ecosystem to Facilitate the Reuse of Medicines and Reduce Medicinal Waste. Pharmacy. 2020; 8(2):58. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmacy8020058
Chicago/Turabian StyleHui, Terence K. L., Bilal Mohammed, Parastou Donyai, Rachel McCrindle, and R. Simon Sherratt. 2020. "Enhancing Pharmaceutical Packaging through a Technology Ecosystem to Facilitate the Reuse of Medicines and Reduce Medicinal Waste" Pharmacy 8, no. 2: 58. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmacy8020058