Regulation of the Photon Spectrum on Growth and Nutritional Attributes of Baby-Leaf Lettuce at Harvest and during Postharvest Storage
Abstract
:1. Introduction
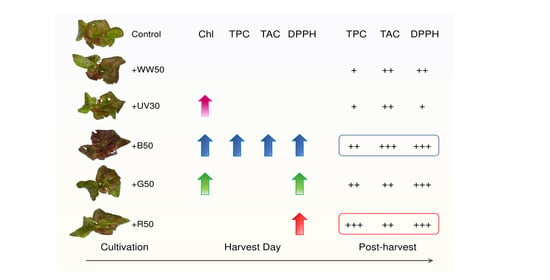
2. Results
2.1. Shoot Weight and Plant Morphology
2.2. Chlorophyll Concentration
2.3. Total Phenolic Content
2.4. Total Anthocyanin Content
2.5. DPPH Free Radical Scavenging Activity
2.6. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Growth Conditions
4.3. Production Culture and Environment
4.4. Lighting Treatments
4.5. Postharvest Storage Conditions
4.6. Biometric Measurements and Water Content Determination
4.7. Non-Destructive Estimation of Chlorophyll Content
4.8. Biochemical Analysis
4.8.1. Quantification of Total Phenolic Content
4.8.2. Quantification of Total Monomeric Anthocyanin Pigment Content
4.8.3. Evaluation of Antiradical Activity by 2.2–Diphenyl–1–Picrylhydrazyl Free Radical (DPPH) Scavenging Activity Method
4.9. Statistical Analysis
4.10. Principal Component Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Iersel, M.W. Optimizing LED lighting in controlled environment agriculture. In Light Emitting Diodes for Agriculture; Springer: Berlin/Heidelberg, Germany, 2017; pp. 59–80. [Google Scholar]
- Benke, K.; Tomkins, B. Future food-production systems: Vertical farming and controlled-environment agriculture. Sustainability 2017, 13, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.D.; Agarwal, A. Light Emitting Diodes for Agriculture; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Xu, Y. Nature and source of light for plant factory. In Plant Factory Using Artificial Light; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–69. [Google Scholar]
- Mitchell, C.A.; Sheibani, F. LED advancements for plant-factory artificial lighting. In Plant Factory; Elsevier: Amsterdam, The Netherlands, 2020; pp. 167–184. [Google Scholar]
- Baslam, M.; Morales, F.; Garmendia, I.; Goicoechea, N. Nutritional quality of outer and inner leaves of green and red pigmented lettuces (Lactuca sativa L.) consumed as salads. Sci. Hortic. 2013, 151, 103–111. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.M.; Vupputuri, S.; Myers, L.; Whelton, P.K. Fruit and vegetable intake and risk of cardiovascular disease in us adults: The first national health and nutrition examination survey epidemiologic follow-up study. Am. J. Clin. Nutr. 2002, 76, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, H.-C.; Joshipura, K.J.; Jiang, R.; Hu, F.B.; Hunter, D.; Smith-Warner, S.A.; Colditz, G.A.; Rosner, B.; Spiegelman, D.; Willett, W.C. Fruit and vegetable intake and risk of major chronic disease. J. Natl. Cancer Inst. 2004, 96, 1577–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Meng, Q.; Boldt, J.; Runkle, E.S. Blue radiation interacts with green radiation to influence growth and predominantly controls quality attributes of lettuce. J. Am. Soc. Hortic. Sci. 2020, 1, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Viršilė, A.; Brazaitytė, A.; Vaštakaitė-Kairienė, V.; Miliauskienė, J.; Jankauskienė, J.; Novičkovas, A.; Laužikė, K.; Samuolienė, G. The distinct impact of multi-color LED light on nitrate, amino acid, soluble sugar and organic acid contents in red and green leaf lettuce cultivated in controlled environment. Food Chem. 2020, 310, 125799. [Google Scholar] [CrossRef]
- Witkowska, I.M. Factors Affecting the Postharvest Performance of Fresh-Cut Lettuce. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2013. [Google Scholar]
- Brecht, J.K. Physiology of lightly processed fruits and vegetables. HortScience 1995, 30, 18–22. [Google Scholar] [CrossRef]
- Deng, M.; Qian, H.; Chen, L.; Sun, B.; Chang, J.; Miao, H.; Cai, C.; Wang, Q. Influence of pre-harvest red light irradiation on main phytochemicals and antioxidant activity of chinese kale sprouts. Food Chem. 2017, 222, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nicole, C.; Mooren, J.; Pereira Terra, A.; Larsen, D.; Woltering, E.; Marcelis, L.; Verdonk, J.; Schouten, R.; Troost, F. Effects of LED lighting recipes on postharvest quality of leafy vegetables grown in a vertical farm. Acta Hortic. 2017, 1256, 481–488. [Google Scholar] [CrossRef]
- Legris, M.; Klose, C.; Burgie, E.S.; Rojas, C.C.R.; Neme, M.; Hiltbrunner, A.; Wigge, P.A.; Schäfer, E.; Vierstra, R.D.; Casal, J.J. Phytochrome B integrates light and temperature signals in arabidopsis. Science 2016, 354, 897–900. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.-H.; Domijan, M.; Klose, C.; Biswas, S.; Ezer, D.; Gao, M.; Khattak, A.K.; Box, M.S.; Charoensawan, V.; Cortijo, S. Phytochromes function as thermosensors in arabidopsis. Science 2016, 354, 886–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paik, I.; Huq, E. Plant Photoreceptors: Multi-Functional Sensory Proteins and Their Signaling Networks; Academic Press: Cambridge, MA, USA, 2019; Volume 92, pp. 114–121. [Google Scholar]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Sun, J.; Nishio, J.N.; Vogelmann, T.C. Green light drives CO2 fixation deep within leaves. Plant Cell Physiol. 1998, 39, 1020–1026. [Google Scholar] [CrossRef]
- Wang, Y.; Folta, K.M. Contributions of green light to plant growth and development. Am. J. Bot. 2013, 100, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Bugbee, B. Economics of LED lighting. In Light Emitting Diodes for Agriculture; Springer: Berlin/Heidelberg, Germany, 2017; pp. 81–99. [Google Scholar]
- Kim, H.-H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-light supplementation for enhanced lettuce growth under red- and blue-light-emitting diodes. HortScience 2004, 39, 1617–1622. [Google Scholar] [CrossRef] [Green Version]
- Johkan, M.; Shoji, K.; Goto, F.; Hahida, S.; Yoshihara, T. Effect of green light wavelength and intensity on photomorphogenesis and photosynthesis in lactuca sativa. Environ. Exp. Bot. 2012, 75, 128–133. [Google Scholar] [CrossRef]
- Mickens, M.; Skoog, E.; Reese, L.; Barnwell, P.; Spencer, L.; Massa, G.; Wheeler, R. A strategic approach for investigating light recipes for ‘outredgeous’ red romaine lettuce using white and monochromatic LEDs. Life Sci. Space Res. 2018, 19, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xue, X.; Guo, W.; Wang, L.; Qiao, X. Growth and nutritional properties of lettuce affected by mixed irradiation of white and supplemental light provided by light-emitting diode. Sci. Hortic. 2016, 200, 111–118. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Growth responses of red-leaf lettuce to temporal spectral changes. Front. Plant Sci. 2020, 11, 1623. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.H.; Benitez, A. Chlorophylls: Analysis in plant materials. In Modern Methods of Plant Analysis/Moderne Methoden der Pflanzenanalyse; Springer: Berlin/Heidelberg, Germany, 1955; pp. 142–196. [Google Scholar]
- Agathokleous, E.; Barceló, D.; Tsatsakis, A.; Calabrese, E.J. Hydrocarbon-induced hormesis: 101 years of evidence at the margin? Environ. Pollut. 2020, 265, 114846. [Google Scholar] [CrossRef]
- Vass, I.; Turcsányi, E.; Touloupakis, E.; Ghanotakis, D.; Petrouleas, V. The mechanism of UV-A radiation-induced inhibition of photosystem II electron transport studied by EPR and chlorophyll fluorescence. Biochemistry 2002, 41, 10200–10208. [Google Scholar] [CrossRef]
- Tyystjärvi, E. Photoinhibition of photosystem II and photodamage of the oxygen evolving manganese cluster. Coord. Chem. Rev. 2008, 252, 361–376. [Google Scholar] [CrossRef]
- Samuolienė, G.; Viršilė, A.; Miliauskienė, J.; Haimi, P.; Laužikė, K.; Jankauskienė, J.; Novičkovas, A.; Kupčinskienė, A.; Brazaitytė, A. The photosynthetic performance of red leaf lettuce under UV-A irradiation. Agronomy 2020, 10, 761. [Google Scholar] [CrossRef]
- Chen, Y.; Li, T.; Yang, Q.; Zhang, Y.; Zou, J.; Bian, Z.; Wen, X. UVA radiation is beneficial for yield and quality of indoor cultivated lettuce. Front. Plant Sci. 2019, 10, 1563. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Zhang, Y.; Song, S.; Su, W.; Hao, Y.; Liu, H. UV-A and FR irradiation improves growth and nutritional properties of lettuce grown in an artificial light plant factory. Food Chem. 2021, 345, 128727. [Google Scholar] [CrossRef] [PubMed]
- Brazaityte, A.; Virsile, A.; Jankauskiene, J.; Sakalauskiene, S.; Samuoliene, G.; Sirtautas, R.; Novickovas, A.; Dabasinskas, L.; Miliauskiene, J.; Vastakaite, V. Effect of supplemental UV-A irradiation in solid-state lighting on the growth and phytochemical content of microgreens. Int. Agrophys. 2015, 29, 13–22. [Google Scholar] [CrossRef]
- Fan, X.; Zang, J.; Xu, Z.; Guo, S.; Jiao, X.; Liu, X.; Gao, Y. Effects of different light quality on growth, chlorophyll concentration and chlorophyll biosynthesis precursors of non-heading chinese cabbage (Brassica campestris L.). Acta Physiol. Plant. 2013, 35, 2721–2726. [Google Scholar] [CrossRef]
- Wang, H.; Gu, M.; Cui, J.; Shi, K.; Zhou, Y.; Yu, J. Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of calvin cycle genes and carbohydrate accumulation in cucumis sativus. J. Photochem. Photobiol. B 2009, 96, 30–37. [Google Scholar] [CrossRef]
- Poudel, P.R.; Kataoka, I.; Mochioka, R. Effect of red-and blue-light-emitting diodes on growth and morphogenesis of grapes. Plant Cell Tissue Organ Cult. 2008, 92, 147–153. [Google Scholar] [CrossRef]
- McCormac, A.C.; Fischer, A.; Kumar, A.M.; Söll, D.; Terry, M.J. Regulation of HEMA1 expression by phytochrome and a plastid signal during de-etiolation in arabidopsis thaliana. Plant J. 2001, 25, 549–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuno, T.; Amaki, W.; Watanabe, H. Effects of monochromatic light irradiation by LED on the growth and anthocyanin contents in leaves of cabbage seedlings. Acta Hortic. 2011, 907, 179–184. [Google Scholar] [CrossRef]
- Li, H.; Tang, C.; Xu, Z.; Liu, X.; Han, X. Effects of different light sources on the growth of non-heading chinese cabbage (Brassica campestris L.). J. Agric. Sci. 2012, 4, 262. [Google Scholar] [CrossRef] [Green Version]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J. Biol. Sci. 2018, 25, 631–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Csepregi, K.; Hideg, É. Phenolic compound diversity explored in the context of photo-oxidative stress protection. Phytochem. Anal. 2018, 29, 129–136. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef] [Green Version]
- Stutte, G.W.; Edney, S.; Skerritt, T. Photoregulation of bioprotectant content of red leaf lettuce with light-emitting diodes. HortScience 2009, 44, 79–82. [Google Scholar] [CrossRef] [Green Version]
- Bian, Z.-H.; Cheng, R.-F.; Yang, Q.-C.; Wang, J.; Lu, C. Continuous light from red, blue, and green light-emitting diodes reduces nitrate content and enhances phytochemical concentrations and antioxidant capacity in lettuce. J. Am. Soc. Hortic. Sci. 2016, 141, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Samuolienė, G.; Urbonavičiūtė, A.; Brazaitytė, A.; Šabajevienė, G.; Sakalauskaitė, J.; Duchovskis, P. The impact of LED illumination on antioxidant properties of sprouted seeds. Open Life Sci. 2011, 6, 68–74. [Google Scholar] [CrossRef]
- D’Souza, C.; Yuk, H.; Khoo, G.H.; Zhou, W. Application of light-emitting diodes in food production, postharvest preservation, and microbiological food safety. Compr. Rev. Food Sci. Food Saf. 2015, 14, 719–740. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using folin–ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the ph differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrolstad, R.E.; Durst, R.W.; Giusti, M.M.; Rodriguez-Saona, L.E. Analysis of anthocyanins in nutraceuticals. In Quality Management of Nutraceuticals; American Chemical Society: Washington, DC, USA, 2002; ISBN 1947-5918. [Google Scholar]
- Sharma, O.P.; Bhat, T.K. DPPH Antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- De Mendiburu, F.; de Mendiburu, M.F. Package ‘Agricolae’; Version 1.3-3; R Package: Madison, WI, USA, 7 June 2020. [Google Scholar]



| Factors | F1 | F2 |
|---|---|---|
| Eigenvalue | 3.278 | 2.380 |
| Variability (%) | 40.97 | 29.75 |
| Cumulative variability (%) | 70.72 | |
| Factor Loadings | ||
| FW | 0.923 | 0.039 |
| DW | 0.868 | 0.084 |
| LL | 0.763 | −0.237 |
| LW | 0.924 | −0.005 |
| SPAD | 0.199 | 0.764 |
| DPPH | 0.390 | 0.463 |
| TAC | −0.140 | 0.867 |
| TPC | −0.155 | 0.875 |
| Factor Scores | ||
| Control | −1.106 | −1.773 |
| +WW50 | 0.237 | −0.595 |
| +UV30 | 0.029 | −0.461 |
| +B50 | −1.110 | 2.974 |
| +G50 | 1.559 | 0.229 |
| +R50 | 0.392 | −0.375 |
| Lighting Treatments | PFD, µmol m−2 s−1 | DTPFDI | |||||
|---|---|---|---|---|---|---|---|
| Warm-White (WW) | UV-A (UV) | Blue (B) | Green (G) | Red (R) | Total | ||
| Control | 200 | 200 | 12.9 | ||||
| +WW50 | 250 | 250 | 16.2 | ||||
| +UV30 | 200 | 30 | 230 | 14.9 | |||
| +B50 | 200 | 50 | 250 | 16.2 | |||
| +G50 | 200 | 50 | 250 | 16.2 | |||
| +R50 | 200 | 50 | 250 | 16.2 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaštakaitė-Kairienė, V.; Kelly, N.; Runkle, E.S. Regulation of the Photon Spectrum on Growth and Nutritional Attributes of Baby-Leaf Lettuce at Harvest and during Postharvest Storage. Plants 2021, 10, 549. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10030549
Vaštakaitė-Kairienė V, Kelly N, Runkle ES. Regulation of the Photon Spectrum on Growth and Nutritional Attributes of Baby-Leaf Lettuce at Harvest and during Postharvest Storage. Plants. 2021; 10(3):549. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10030549
Chicago/Turabian StyleVaštakaitė-Kairienė, Viktorija, Nathan Kelly, and Erik S. Runkle. 2021. "Regulation of the Photon Spectrum on Growth and Nutritional Attributes of Baby-Leaf Lettuce at Harvest and during Postharvest Storage" Plants 10, no. 3: 549. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10030549