Essential Oils of Sage, Rosemary, and Bay Laurel Inhibit the Life Stages of Oomycete Pathogens Important in Aquaculture
Abstract
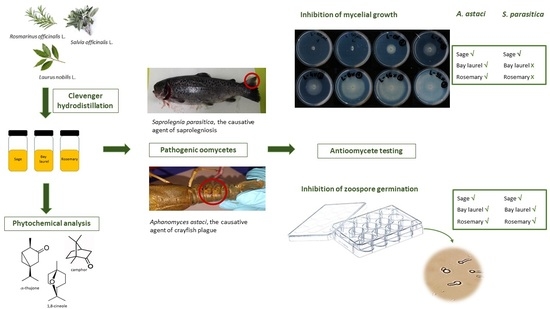
:1. Introduction
2. Results
2.1. Chemical Composition of Essential Oils
2.2. Inhibition of Mycelial Growth
2.3. Inhibition of Zoospore Germination
2.4. Correlation of the Observed Inhibitory Effects and Representation of Different Volatiles in the Essential Oils Studied
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Microorganisms
5.2. Plant Material and Essential Oil Isolation
5.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
5.4. Testing of Anti-Oomycete Activity of Essential Oils
5.4.1. Inhibition of Mycelial Growth
5.4.2. Inhibition of Zoospore Germination
5.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van den Berg, A.H.; McLaggan, D.; Diéguez-Uribeondo, J.; van West, P. The impact of the water moulds Saprolegnia diclina and Saprolegnia parasitica on natural ecosystems and the aquaculture industry. Fungal Biol. Rev. 2013, 27, 33–42. [Google Scholar] [CrossRef]
- Harlioǧlu, M.M. The harvest of the freshwater crayfish Astacus leptodactylus Eschscholtz in Turkey: Harvest history, impact of crayfish plague, and present distribution of harvested populations. Aquac. Int. 2008, 16, 351–360. [Google Scholar] [CrossRef]
- Holdich, D.M. A review of astaciculture: Freshwater crayfish farming. Aquat. Living Resour. 1993, 6, 307–317. [Google Scholar] [CrossRef]
- Souty-Grosset, C.; Reynolds, J.D. Current ideas on methodological approaches in European crayfish conservation and restocking procedures. Knowl. Manag. Aquat. Ecosyst. 2009, 1, 394–395. [Google Scholar] [CrossRef]
- Sarowar, M.N.; Hossain, M.J.; Nasrin, T.; Naznin, T.; Hossain, Z.; Rahman, M.M. Molecular identification of oomycete species affecting aquaculture in Bangladesh. Aquac. Fish. 2019, 4, 105–113. [Google Scholar] [CrossRef]
- Stueland, S.; Hatai, K.; Skaar, I. Morphological and physiological characteristics of Saprolegnia spp. strains pathogenic to Atlantic salmon, Salmo salar L. J. Fish Dis. 2005, 28, 445–453. [Google Scholar] [CrossRef]
- Thoen, E.; Evensen, Ø.; Skaar, I. Pathogenicity of Saprolegnia spp. to Atlantic salmon, Salmo salar L., eggs. J. Fish Dis. 2011, 34, 601–608. [Google Scholar] [CrossRef]
- Diéguez-Uribeondo, J.; Cerenius, L.; Söderhäll, K. Saprolegnia parasitica and its virulence on three different species of freshwater crayfish. Aquaculture 1994, 120, 219–228. [Google Scholar] [CrossRef]
- Liu, Y.; De Bruijn, I.; Jack, A.L.; Drynan, K.; van Den Berg, A.H.; Thoen, E.; Sandoval-Sierra, V.; Skaar, I.; van West, P.; Diéguez-Uribeondo, J.; et al. Deciphering microbial landscapes of fish eggs to mitigate emerging diseases. ISME J. 2014, 8, 2002–2014. [Google Scholar] [CrossRef]
- Edsman, L.; Nyström, P.; Sandström, A.; Stenberg, M.; Kokko, H.; Tiitinen, V.; Makkonen, J.; Jussila, J. Eroded swimmeret syndrome in female crayfish Pacifastacus leniusculus associated with Aphanomyces astaci and Fusarium spp. infections. Dis. Aquat. Organ. 2015, 112, 219–228. [Google Scholar] [CrossRef]
- Kokko, H.; Koistinen, L.; Harlioǧlu, M.M.; Makkonen, J.; Aydin, H.; Jussila, J. Des populations turques d’écrevisses à pattes grêles (Astacus leptodactylus) productives porteuses d’Aphanomyces astaci. Knowl. Manag. Aquat. Ecosyst. 2012, 404, 12. [Google Scholar] [CrossRef] [Green Version]
- Aydin, H.; Kokko, H.; Makkonen, J.; Kortet, R.; Kukkonen, H.; Jussila, J. The signal crayfish is vulnerable to both the As and the PsI-isolates of the crayfish plague. Knowl. Manag. Aquat. Ecosyst. 2014, 413, 03. [Google Scholar] [CrossRef] [Green Version]
- Sandström, A.; Andersson, M.; Asp, A.; Bohman, P.; Edsman, L.; Engdahl, F.; Nyström, P.; Stenberg, M.; Hertonsson, P.; Vrålstad, T.; et al. Population collapses in introduced non-indigenous crayfish. Biol. Invasions 2014, 16, 1961–1977. [Google Scholar] [CrossRef]
- Edgerton, B.F.; Evans, L.H.; Stephens, F.J.; Overstreet, R.M. Review article: Synopsis of freshwater crayfish diseases and commensal organisms. Aquaculture 2002, 206, 57–135. [Google Scholar] [CrossRef] [Green Version]
- EC. Council Regulation 2377/90/EEC of 26 June 1990 on laying down a Community procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuff of animal origin. Off. J. Eur. Commun. 1990, 224, 1–12. [Google Scholar]
- Marking, L.L.; Rach, J.J.; Schreier, T.M. American Fisheries Society Evaluation of Antifungal Agents for Fish Culture. Progress. Fish-Culturist 1994, 56, 225–231. [Google Scholar] [CrossRef]
- Meyer, F.P.; Jorgenson, T.A. Teratological and Other Effects of Malachite Green on Development of Rainbow Trout and Rabbits. Trans. Am. Fish. Soc. 1983, 112, 818–824. [Google Scholar] [CrossRef]
- Panandiker, A.; Fernandes, C.; Rao, K.V.K. The cytotoxic properties of malachite green are associated with the increased demethylase, aryl hydrocarbon hydroxylase and lipid peroxidation in primary cultures of Syrian hamster embryo cells. Cancer Lett. 1992, 67, 93–101. [Google Scholar] [CrossRef]
- Srivastava, S.; Sinha, R.; Roy, D. Toxicological effects of malachite green. Aquat. Toxicol. 2004, 66, 319–329. [Google Scholar] [CrossRef]
- Wooster, G.; Martinez, C.; Bowser, P.; O’Hara, D. Human Health Risks Associated with Formalin Treatments Used in Aquaculture: Initial Study. N. Am. J. Aquac. 2005, 67, 111–113. [Google Scholar] [CrossRef]
- Norliana, S.; Abdulamir, A.S.; Abu Bakar, F.; Salleh, A.B. The health risk of formaldehyde to human beings. Am. J. Pharmacol. Toxicol. 2009, 4, 98–106. [Google Scholar] [CrossRef]
- Cui, N.; Zhang, X.; Xie, Q.; Wang, S.; Chen, J.; Huang, L.; Qiao, X.; Li, X.; Cai, X. Toxicity profile of labile preservative bronopol in water: The role of more persistent and toxic transformation products. Environ. Pollut. 2011, 159, 609–615. [Google Scholar] [CrossRef]
- Jacob, A.P.; Culver, D.A.; Lanno, R.P.; Voigt, A. Ecological impacts of fluridone and copper sulphate in catfish aquaculture ponds. Environ. Toxicol. Chem. 2016, 35, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Jussila, J.; Toljamo, A.; Makkonen, J.; Kukkonen, H.; Kokko, H. Practical disinfection chemicals for fishing and crayfishing gear against crayfish plague transfer. Knowl. Manag. Aquat. Ecosyst. 2014, 413, 02. [Google Scholar] [CrossRef] [Green Version]
- Afzali, S.F.; Wong, W.L. In vitro screening of Sonneratia alba extract against the oomycete fish pathogen, Aphanomyces invadans. Iran. J. Fish. Sci. 2017, 16, 1333–1340. [Google Scholar] [CrossRef]
- Borisutpeth, M.; Kanbutra, P.; Weerakhun, S.; Wada, S.; Hatai, K. In vitro antifungal activity of Cassia fistula L. against selected pathogenic water molds. Int. J. Phytomed. 2014, 6, 237–242. [Google Scholar] [CrossRef]
- Borisutpeth, P.; Kanbutra, P.; Hanjavanit, C.; Chukanhom, K.; Funaki, D.; Hatai, K. Effects of Thai Herbs on the Control of Fungal Infection in Tilapia Eggs and the Toxicity to the Eggs. Aquac. Sci. 2009, 57, 475–482. [Google Scholar] [CrossRef]
- Campbell, R.E.; Lilley, J.H.; Taukhid; Panyawachira, V.; Kanchanakhan, S. In vitro screening of novel treatments for Aphanomyces invadans. Aquac. Res. 2001, 32, 223–233. [Google Scholar] [CrossRef]
- Pagliarulo, C.; Sateriale, D.; Scioscia, E.; De Tommasi, N.; Colicchio, R.; Pagliuca, C.; Scaglione, E.; Jussila, J.; Makkonen, J.; Salvatore, P.; et al. Growth, survival and spore formation of the pathogenic aquatic oomycete Aphanomyces astaci and Fungus fusarium avenaceum are inhibited by Zanthoxylum rhoifolium bark extracts in vitro. Fishes 2018, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Tandel, R.S.; Chadha, N.K.; Dash, P.; Sawant, P.B.; Pandey, N.N.; Chandra, S.; Bhat, R.A.H.; Thakuria, D. An in-vitro study of Himalayan plant extracts against oomycetes disease Saprolegniasis in rainbow trout (Oncorhynchus mykiss). J. Environ. Biol. 2021, 42, 1008–1018. [Google Scholar] [CrossRef]
- Caruana, S.; Yoon, G.H.; Freeman, M.A.; Mackie, J.A.; Shinn, A.P. The efficacy of selected plant extracts and bioflavonoids in controlling infections of Saprolegnia australis (Saprolegniales; Oomycetes). Aquaculture 2012, 358–359, 146–154. [Google Scholar] [CrossRef]
- Gormez, O.; Diler, O. In vitro antifungal activity of essential oils from Tymbra, Origanum, Satureja species and some pure compounds on the fish pathogenic fungus, Saprolegnia parasitica. Aquac. Res. 2014, 45, 1196–1201. [Google Scholar] [CrossRef]
- Hoskonen, P.; Heikkinen, J.; Eskelinen, P.; Pirhonen, J. Efficacy of clove oil and ethanol against Saprolegnia sp. and usability as antifungal agents during incubation of rainbow trout Oncorhynchus mykiss (Walbaum) eggs. Aquac. Res. 2015, 46, 581–589. [Google Scholar] [CrossRef]
- Khosravi, A.R.; Shokri, H.; Sharifrohani, M.; Mousavi, H.E.; Moosavi, Z. Evaluation of the antifungal activity of Zataria multiflora, Geranium herbarium, and Eucalyptus camaldolensis essential oils on Saprolegnia parasitica-infected rainbow trout (Oncorhynchus mykiss) eggs. Foodborne Pathog. Dis. 2012, 9, 674–679. [Google Scholar] [CrossRef] [Green Version]
- Madrid, A.; Godoy, P.; González, S.; Zaror, L.; Moller, A.; Werner, E.; Cuellar, M.; Villena, J.; Montenegro, I. Chemical characterization and anti-oomycete activity of Laureliopsis philippianna essential oils against Saprolegnia parasitica and S. australis. Molecules 2015, 20, 8033–8047. [Google Scholar] [CrossRef] [Green Version]
- Metin, S.; Diler, O.; Didinen, B.I.; Terzioglu, S.; Gormez, O. In vitro and in vivo antifungal activity of Satureja cuneifolia ten essential oil on Saprolegnia parasitica strains isolated from rainbow trout (Oncorhynchus mykiss, Walbaum) eggs. Aquac. Res. 2015, 46, 1396–1402. [Google Scholar] [CrossRef]
- Nardoni, S.; Najar, B.; Fronte, B.; Pistelli, L.; Mancianti, F. In vitro activity of essential oils against Saprolegnia parasitica. Molecules 2019, 24, 1270. [Google Scholar] [CrossRef] [Green Version]
- Pirbalouti, A.G.; Taheri, M.; Raisee, M.; Bahrami, H.R.; Abdizadeh, R. In vitro antifungal activity of plant extracts on Saprolegnia parasitica from cutaneous lesions of rainbow trout (Oncorhynchus mykiss) eggs. J. Food Agric. Environ. 2009, 7, 94–96. [Google Scholar]
- Saleh, M.; Soltani, M.; Islami, H.R. In vitro antifungal activity of some essential oils against some filamentous fungi of rainbow trout (Oncorhynchus mykiss) eggs. AACL Bioflux 2015, 8, 367–380. [Google Scholar]
- Parikh, L.; Agindotan, B.O.; Burrows, M.E. Antifungal Activity of Plant-Derived Essential Oils on Pathogens of Pulse Crops. Plant Dis. 2021. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, N.; Fu, Y.J.; Wang, W.; Luo, M.; Zhao, C.J.; Zu, Y.G.; Liu, X.L. Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environ. Toxicol. Pharmacol. 2011, 32, 63–68. [Google Scholar] [CrossRef]
- Özçakmak, S.; Muhammet, D.; Azime, Y. Antifungal activity of lemon balm and sage essential oils on the growth of ochratoxigenic Penicillium verrucosum. Afr. J. Microbiol. Res. 2012, 6, 3079–3084. [Google Scholar] [CrossRef] [Green Version]
- Fidan, H.; Stefanova, G.; Kostova, I.; Stankov, S.; Damyanova, S.; Stoyanova, A.; Zheljazkov, V.D. Chemical Composition and Antimicrobial Activity of Laurus nobilis L. Essential oils from Bulgaria. Molecules 2019, 24, 804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tampieri, M.P.; Galuppi, R.; Carelle, M.S.; Macchioni, F.; Cioni, P.L.; Morelli, I. Effect of selected essential oils and pure compounds on Saprolegnia parasitica. Pharm. Biol. 2003, 41, 584–591. [Google Scholar] [CrossRef] [Green Version]
- Klancnik, A.; Guzej, B.; Kolar, M.H.; Abramovic, H.; Mozina, S.S. In vitro antimicrobial and antioxidant activity of commercial rosemary extract formulations. J. Food Prot. 2009, 72, 1174–1752. [Google Scholar] [CrossRef]
- Stević, T.; Berić, T.; Šavikin, K.; Soković, M.; Gođevac, D.; Dimkić, I.; Stanković, S. Antifungal activity of selected essential oils against fungi isolated from medicinal plant. Ind. Crops Prod. 2014, 55, 116–122. [Google Scholar] [CrossRef]
- Hu, J.; Hong, C.; Stromberg, E.L.; Moorman, G.W. Effects of propamocarb hydrochloride on mycelial growth, sporulation, and infection by Phytophthora nicotianae isolates from Virginia nurseries. Plant Dis. 2007, 91, 414–420. [Google Scholar] [CrossRef]
- Lawrence, S.A.; Armstrong, C.B.; Patrick, W.M.; Gerth, M.L. High-throughput chemical screening identifies compounds that inhibit different stages of the Phytophthora agathidicida and Phytophthora cinnamomi life cycles. Front. Microbiol. 2017, 8, 1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miljanović, A.; Bielen, A.; Grbin, D.; Marijanović, Z.; Andlar, M.; Rezić, T.; Roca, S.; Jerković, I.; Vikić-Topić, D.; Dent, M. Effect of enzymatic, ultrasound, and reflux extraction pretreatments on the chemical composition of essential oils. Molecules 2020, 25, 4818. [Google Scholar] [CrossRef] [PubMed]
- Boulila, A.; Hassen, I.; Haouari, L.; Mejri, F.; Amor, I.B.; Casabianca, H.; Hosni, K. Enzyme-assisted extraction of bioactive compounds from bay leaves (Laurus nobilis L.). Ind. Crops Prod. 2015, 74, 485–493. [Google Scholar] [CrossRef]
- Hatipoglu, S.D.; Zorlu, N.; Dirmenci, T.; Goren, A.C.; Ozturk, T.; Topcu, G. Determination of volatile organic compounds in fourty five salvia species by thermal desorption-GC-MS technique. Rec. Nat. Prod. 2016, 10, 659–700. [Google Scholar]
- Hosni, K.; Hassen, I.; Chaâbane, H.; Jemli, M.; Dallali, S.; Sebei, H.; Casabianca, H. Enzyme-assisted extraction of essential oils from thyme (Thymus capitatus L.) and rosemary (Rosmarinus officinalis L.): Impact on yield, chemical composition and antimicrobial activity. Ind. Crops Prod. 2013, 47, 291–299. [Google Scholar] [CrossRef]
- Olmedo, R.H.; Asensio, C.M.; Grosso, N.R. Thermal stability and antioxidant activity of essential oils from aromatic plants farmed in Argentina. Ind. Crops Prod. 2015, 69, 21–28. [Google Scholar] [CrossRef]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem. Toxicol. 2013, 55, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, P.; Beraldo, P.; Massimo, M.; Fioravanti, M.L.; Volpatti, M.; Dirks, R.; Galuppi, R. Comparative Therapeutic Effects of Natural Compounds Against Saprolegnia spp. (Oomycota) and Amyloodinium ocellatum (Dinophyceae). Front. Vet. Sci. 2020, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Teker, T.; Sefer, Ö.; Gazdağlı, A.; Yörük, E.; Varol, G.İ.; Albayrak, G. α-Thujone exhibits an antifungal activity against F. graminearum by inducing oxidative stress, apoptosis, epigenetics alterations and reduced toxin synthesis. Eur. J. Plant Pathol. 2021, 160, 611–622. [Google Scholar] [CrossRef]
- Gazdağlı, A.; Sefer, Ö.; Yörük, E.; Varol, G.İ.; Teker, T.; Albayrak, G. Investigation of Camphor Effects on Fusarium graminearum and F. culmorum at Different Molecular Levels. Pathogens 2018, 7, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agus, H.H.; Sengoz, C.O.; Yilmaz, S. Oxidative stress-mediated apoptotic cell death induced by camphor in sod1-deficient Schizosaccharomyces pombe. Toxicol. Res. 2019, 8, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Agus, H.H.; Kok, G.; Derinoz, E.; Oncel, D.; Yilmaz, S. Involvement of Pca1 in ROS-mediated apoptotic cell death induced by alpha-thujone in the fission yeast (Schizosaccharomyces pombe). FEMS Yeast Res. 2020, 20, foaa022. [Google Scholar] [CrossRef]
- Sönmez, A.Y.; Bilen, S.; Albayrak, M.; Yilmaz, S.; Biswas, G.; Hisar, O.; Yanik, T. Effects of Dietary Supplementation of Herbal Oils Containing 1,8-cineole, Carvacrol or Pulegone on Growth Performance, Survival, Fatty Acid Composition, and Liver and Kidney Histology of Rainbow Trout (Oncorhynchus mykiss) Fingerlings. Turk. J. Fish. Aquat. Sci. 2015, 15, 813–819. [Google Scholar] [CrossRef]
- Sari, A.B.; Ustuner-Aydal, O. Antioxidant and Immunostimulant Effects of Origanum Minutiflorum O. Schwarz Et. Ph Davis in Rainbow Trout. Fresenius Environ. Bull. 2018, 27, 1013–1021. [Google Scholar]
- Yogeshwari, G.; Jagruthi, C.; Anbazahan, S.M.; Mari, L.S.S.; Selvanathan, J.; Arockiaraj, J.; Dhayanithi, N.B.; Ajithkumar, T.T.; Balasundaram, C.; Ramasamy, H. Herbal supplementation diet on immune response in Labeo rohita against Aphanomyces invadans. Aquaculture 2015, 437, 351–359. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Balasundaram, C.; Bhuvaneswari, R. Restorative effect of Azadirachta indicab aqueous leaf extract dip treatment on haematological parameter changes in Cyprinus carpio (L.) experimentally infected with Aphanomyces invadans fungus. J. Appl. Ichthyol. 2005, 21, 410–413. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Lange, B.M.; Croteau, R. Genetic engineering of essential oil production in mint. Curr. Opin. Plant Biol. 1999, 2, 139–144. [Google Scholar] [CrossRef]
- Stevanović, Z.D.; Bošnjak-Neumüller, J.; Pajić-Lijaković, I.; Raj, J.; Vasiljević, M. Essential oils as feed additives—Future perspectives. Molecules 2018, 23, 1717. [Google Scholar] [CrossRef] [Green Version]
- Makkonen, J.; Jussila, J.; Kortet, R.; Vainikka, A.; Kokko, H. Differing virulence of Aphanomyces astaci isolates and elevated resistance of noble crayfish Astacus astacus against crayfish plague. Dis. Aquat. Organ. 2012, 102, 129–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jussila, J.; Kokko, H.; Kortet, R.; Makkonen, J. Aphanomyces astaci PsI-genotype isolates from different Finnish signal crayfish stocks show variation in their virulence but still kill fast. Knowl. Manag. Aquat. Ecosyst. 2013, 411, 10. [Google Scholar] [CrossRef] [Green Version]
- Unestam, T. Studies on the Crayfish Plague Fungus Aphanomyces astaci I. Some Factors Affecting Growth in vitro. Physiol. Plant. 1965, 18, 483–505. [Google Scholar] [CrossRef]
- Zahran, E.; Noga, E.J. Evidence for synergism of the antimicrobial peptide piscidin 2 with antiparasitic and antioomycete drugs. J. Fish Dis. 2010, 33, 995–1003. [Google Scholar] [CrossRef]
- Diéguez-Uribeondo, J.; Cerenius, L.; Söderhäll, K. Repeated zoospore emergence in Saprolegnia parasitica. Mycol. Res. 1994, 98, 810–815. [Google Scholar] [CrossRef]
- Häll, L.; Unestam, T. The effect of fungicides on survival of the crayfish plague fungus, Aphanomyces astaci, Oomycetes, growing on fish scales. Mycopathologia 1980, 72, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Svensson, E.; Unestam, T. Differectial Induction of Zoospore Encystment and Germination in Aphanomyces astaci, Oomycetes. Physiol. Plant. 1975, 35, 210–216. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Development Core Team: Vienna, Austria, 2017. [Google Scholar]
- Sanchez, G. R Package Plsdepot PLS Regression 1. 2012, pp. 1–13. Available online: https://cran.r-project.org/web/packages/plsdepot/plsdepot.pdf (accessed on 15 May 2021).




| EC50 for Mycelium Growth (µL/mL) | EC50 for Zoospore Germination (µL/mL) | |||
|---|---|---|---|---|
| A. astaci | S. parasitica | A. astaci | S. parasitica | |
| Rosemary essential oil Sage essential oil Bay laurel essential oil | 0.060 | N.D. * | 0.049 | 0.063 |
| 0.031 | 0.040 | 0.007 | 0.012 | |
| 0.098 | N.D. * | 0.015 | 0.013 | |
| µg/mL | µg/mL | |||
| Malachite green (pos. control) | 0.020 | 0.120 | 0.020 | 0.032 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miljanović, A.; Grbin, D.; Pavić, D.; Dent, M.; Jerković, I.; Marijanović, Z.; Bielen, A. Essential Oils of Sage, Rosemary, and Bay Laurel Inhibit the Life Stages of Oomycete Pathogens Important in Aquaculture. Plants 2021, 10, 1676. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10081676
Miljanović A, Grbin D, Pavić D, Dent M, Jerković I, Marijanović Z, Bielen A. Essential Oils of Sage, Rosemary, and Bay Laurel Inhibit the Life Stages of Oomycete Pathogens Important in Aquaculture. Plants. 2021; 10(8):1676. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10081676
Chicago/Turabian StyleMiljanović, Anđela, Dorotea Grbin, Dora Pavić, Maja Dent, Igor Jerković, Zvonimir Marijanović, and Ana Bielen. 2021. "Essential Oils of Sage, Rosemary, and Bay Laurel Inhibit the Life Stages of Oomycete Pathogens Important in Aquaculture" Plants 10, no. 8: 1676. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10081676