Metabolomics-Driven Discovery of an Introduced Species and Two Malaysian Piper betle L. Variants
Abstract
:1. Introduction
2. Results and Discussion
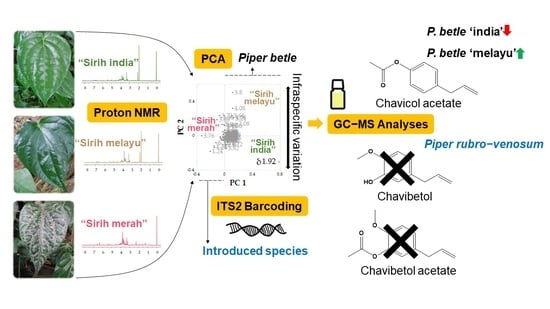
2.1. Principal Components Analysis of 1H-NMR Spectra of Three “Sirih” Variants
2.2. Identification of “Sirih Merah” as P. rubro-venosum hort. ex Rodigas
2.3. Leaf Essential Oil Profiles of P. betle Variants and P. rubro-venosum
2.4. Principal Components Analysis of GC-MS Data of P. betle Leaf Essential Oils
2.5. Hypotheses
3. Materials and Methods
3.1. Chemicals and Disposables
3.2. 1H-NMR Metabolomics of “Sirih” Leaf Extracts
3.2.1. Collection of Plant Materials
3.2.2. Preparation of Samples and Extracts
3.2.3. Acquisition of 1H-NMR Spectra
3.2.4. Preprocessing of 1H-NMR Spectra
3.3. DNA Barcoding of “Sirih Merah”
3.4. GC-MS Metabolomics of Leaf Essential Oils
3.4.1. Collection of Plant Materials
3.4.2. Hydrodistillation of Plant Materials
3.4.3. GC-MS Analyses
GC-MS Analysis I
GC-MS Analysis II
3.4.4. Preprocessing of GC-MS Data
3.5. Welch’s Analysis of Variance (ANOVA) and Principal Components Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patra, A.P.; Mukherjee, A.K.; Acharya, L. Comparative study of RAPD and ISSR markers to assess the genetic diversity of betel vine (Piper betle L.) in Orissa, India. Am. J. Biochem. Mol. Biol. 2011, 1, 200–211. [Google Scholar] [CrossRef]
- Ong, H.C.; Nordiana, M. Malay ethno-medico botany in Machang, Kelantan, Malaysia. Fitoterapia 1999, 70, 502–513. [Google Scholar] [CrossRef]
- Jaramillo, M.A.; Callejas, R.; Davidson, C.; Smith, J.F.; Stevens, A.C.; Tepe, E.J. A phylogeny of the tropical genus Piper using ITS and the chloroplast intron psbJ-petA. Syst. Bot. 2008, 33, 647–660. [Google Scholar] [CrossRef]
- Asmarayani, R. Phylogenetic relationships in Malesian-Pacific Piper (Piperaceae) and their implications for systematics. Taxon 2018, 67, 693–724. [Google Scholar] [CrossRef]
- van Welzen, P.C.; Parnell, J.A.N.; Slik, J.W.F. Wallace’s line and plant distributions: Two or three phytogeographical areas and where to group Java? Biol. J. Linn. Soc. 2011, 103, 531–545. [Google Scholar] [CrossRef]
- Adam, N.S.; Abd Rahman, R.; Abu Bakar, A.F.; Zainal, N.F.; Safie, S.Z.; Mansor, M. Distribution of herbs cultivation in Peninsular Malaysia. In Proceedings of the Prosiding Persidangan Industri Herba (Proceedings of Herbal Industry Conference), Putrajaya, Malaysia, 3–5 November 2015; Osman, A., Lim, H.F., Eds.; Forest Research Institute Malaysia: Kepong, Malaysia, 2015; pp. 318–323. [Google Scholar]
- Malaysian Herbal Monograph Committee. Piper betle L. (Leaves). In Malaysian Herbal Monograph; Institute for Medical Research: Kuala Lumpur, Malaysia, 2015; pp. 416–427. [Google Scholar]
- Mat Piah, H.; Mustapha, N.M. Glosari II: Istilah botani dan saintifik. In Kitab Tib MSS 1292 PNM edisi dan Suntingan Teks; Forest Research Institute Malaysia (FRIM): Kepong, Malaysia, 2019; p. 242. [Google Scholar]
- Casañas, F.; Simó, J.; Casals, J.; Prohens, J. Toward an evolved concept of landrace. Front. Plant Sci. 2017, 8, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajpai, V.; Pandey, R.; Negi, M.P.S.; Bindu, K.; Kumar, N.; Kumar, B. Characteristic differences in metabolite profile in male and female plants of dioecious Piper betle L. J. Biosci. 2012, 37, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Bhatnagar, S.P.; Sinha, B.N.; Lal, U.R. Pharmacognostic specifications of eight cultivars of Piper betle from eastern region of India. Pharmacogn. J. 2013, 5, 176–183. [Google Scholar] [CrossRef]
- Karak, S.; Bhattacharya, P.; Nandy, A.; Saha, A.; De, B. Metabolite profiling and chemometric study for varietal difference in Piper betle L. leaf. Curr. Metab. 2016, 4, 129–140. [Google Scholar] [CrossRef]
- Islam, M.A.; Ryu, K.Y.; Khan, N.; Song, O.Y.; Jeong, J.Y.; Son, J.H.; Jamila, N.; Kim, K.S. Determination of the volatile compounds in five varieties of Piper betle L. from Bangladesh using simultaneous distillation extraction and gas chromatography/mass spectrometry (SDE-GC/MS). Anal. Lett. 2020, 53, 2413–2430. [Google Scholar] [CrossRef]
- Guha, P.; Nandi, S. Essential oil of betel leaf (Piper betle L.): A novel addition to the world food sector. In Essential Oil Research Trends in Biosynthesis, Analytics, Industrial Applications and Biotechnological Production; Malik, S., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 149–196. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Centering and scaling in component analysis. J. Chemom. 2003, 17, 16–33. [Google Scholar] [CrossRef]
- Hartini, Y.S.; Nugroho, L. The accumulation of two neolignan in the leaves, stems, and flower of red betel (Piper crocatum Ruiz & Pav.). J. Phys. Conf. Ser. 2017, 835, 2–6. [Google Scholar] [CrossRef] [Green Version]
- Li, H.X.; Widowati, W.; Azis, R.; Yang, S.Y.; Kim, Y.H.; Li, W. Chemical constituents of the Piper crocatum leaves and their chemotaxonomic significance. Biochem. Syst. Ecol. 2019, 86, 103905. [Google Scholar] [CrossRef]
- Pandey, R.; Chandra, P.; Srivastva, M.; Arya, K.R.; Shukla, P.K.; Kumar, B. A rapid analytical method for characterization and simultaneous quantitative determination of phytoconstituents in Piper betle landraces using UPLC-ESI-MS/MS. Anal. Methods 2014, 6, 7349–7360. [Google Scholar] [CrossRef]
- Kemprai, P.; Bora, P.K.; Mahanta, B.P.; Sut, D.; Saikia, S.P.; Banik, D.; Haldar, S. Piper betleoides C. DC.: Edible source of betel-scented sesquiterpene-rich essential oil. Flavour Fragr. J. 2020, 35, 70–78. [Google Scholar] [CrossRef]
- Kemprai, P.; Protim Mahanta, B.; Kumar Bora, P.; Jyoti Das, D.; Lakshmi Hati Boruah, J.; Proteem Saikia, S.; Haldar, S. A 1H NMR spectroscopic method for the quantification of propenylbenzenes in the essential oils: Evaluation of key odorants, antioxidants and post-harvest drying techniques for Piper betle L. Food Chem. 2020, 331, 127278. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.J.; Go, Y.; Zhou, H.Q.; Li, H.X.; Lee, S.J.; Park, Y.J.; Widowatib, W.; Rizal, R.; Kim, Y.H.; Yang, S.Y.; et al. Unusual bicyclo[3.2.1]octanoid neolignans from leaves of Piper crocatum and their effect on pyruvate dehydrogenase activity. Plants 2021, 10, 1855. [Google Scholar] [CrossRef] [PubMed]
- Chaveerach, A.; Mokkamul, P.; Sudmoon, R.; Tanee, T. Ethnobotany of the genus Piper (Piperaceae) in Thailand. Ethnobot. Res. Appl. 2006, 4, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Chaveerach, A.; Mokkamul, P.; Sudmoon, R.; Tanee, T. Two new species of Piper (Piperaceae) from Malay Peninsula. Taiwania 2007, 52, 210–215. [Google Scholar] [CrossRef]
- Li, H.X.; Yang, S.Y.; Kim, Y.H.; Li, W. Isolation of two new compounds and other constituents from leaves of Piper crocatum and study of their soluble epoxide hydrolase activities. Molecules 2019, 24, 489. [Google Scholar] [CrossRef] [Green Version]
- Rajudin, E.; Fernando, A.; Yuliandari, R.; Rullah, K.; Indrayani, N.R.; Susanty, A.; Yerti, R.; Ahmad, F.; Mohd Sirat, H.; Arbain, D. Cytotoxic activities of fractions and two isolated compounds from sirih merah (Indonesian red betel), Piper crocatum Ruiz & Pav. Procedia Chem. 2014, 13, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Sanubol, A.; Chaveerach, A.; Sudmoon, R.; Tanee, T.; Noikotr, K.; Chuachan, C. Betel-like-scented Piper plants as diverse sources of industrial and medicinal aromatic chemicals. Chiang Mai J. Sci. 2014, 41, 1171–1181. Available online: https://epg.science.cmu.ac.th/ejournal/journal-detail.php?id=5278 (accessed on 3 August 2021).
- Sanubol, A.; Chaveerach, A.; Tanee, T.; Sudmoon, R. Pre-clinical evaluation of extracts and essential oils from betel-like scent Piper species identified potential cancer treatment. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 89–102. [Google Scholar] [CrossRef] [Green Version]
- Vertical Garden Patrick Blanc: Leaf Colors and Structures 2. Available online: https://www.verticalgardenpatrickblanc.com/inspiration/leaf-colors-and-structures-2?page=3 (accessed on 17 February 2021).
- Sulianti, S.B.; Chairul, D. Perbandingan komponen kimia penyusun minyak atsiri sirih liar (Piper ornatum) yang berasal dari Sulawesi Selatan dan Pulau Seram dengan sirih biasa (Piper betle). Ber. Biologi 2002, 6, 493–499. [Google Scholar] [CrossRef]
- Tnah, L.H.; Lee, S.L.; Tan, A.L.; Lee, C.T.; Ng, K.K.S.; Ng, C.H.; Zakaria, N.F. DNA barcode database of common herbal plants in the tropics: A resource for herbal product authentication. Food Control 2019, 95, 318–326. [Google Scholar] [CrossRef]
- Turner, I.M. A catalogue of the vascular plants of Malaya (part 2 of 2). Gard. Bull. Singapore 1995, 47, 399–403. Available online: https://www.biodiversitylibrary.org/page/43660944#page/67/mode/1up (accessed on 25 April 2019).
- Matyushin, D.D.; Sholokhova, A.Y.; Karnaeva, A.E.; Buryak, A.K. Various aspects of retention index usage for GC-MS library search: A statistical investigation using a diverse data set. Chemometr. Intell. Lab. Syst. 2020, 202, 104042. [Google Scholar] [CrossRef]
- Samokhin, A.; Sotnezova, K.; Lashin, V.; Revelsky, I. Evaluation of mass spectral library search algorithms implemented in commercial software. J. Mass Spectrom. 2015, 50, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Babushok, V.I.; Andriamaharavo, N.R. Use of large retention index database for filtering of GC-MS false positive identifications of compounds. Chromatographia 2012, 75, 685–692. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef] [Green Version]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2017. [Google Scholar]
- García, D.; Alvarez, A.; Tornos, P.; Fernandez, A.; Sáenz, T. Gas chromatographic-mass spectrometry study of the essential oils of Pimenta racemosa var. terebinthina and P. racemosa var. grisea. Z. Naturforsch. C 2002, 57, 449–451. [Google Scholar] [CrossRef]
- Cardeal, Z.L.; Gomes da Silva, M.D.R.; Marriott, P.J. Comprehensive two-dimensional gas chromatography/ mass spectrometric analysis of pepper volatiles. Rapid Commun. Mass Spectrom. 2006, 20, 2823–2836. [Google Scholar] [CrossRef] [PubMed]
- Yassaa, N.; Meklati, B.Y.; Cecinato, A. Evaluation of monoterpenic biogenic volatile organic compounds in ambient air around Eucalyptus globulus, Pinus halepensis and Cedrus atlantica trees growing in Algiers city area by chiral and achiral capillary gas chromatography. Atmos. Environ. 2000, 34, 2809–2816. [Google Scholar] [CrossRef]
- Perez-Cacho, P.R.; Mahattanatawee, K.; Smoot, J.M.; Rouseff, R. Identification of sulfur volatiles in canned orange juices lacking orange flavor. J. Agric. Food Chem. 2007, 55, 5761–5767. [Google Scholar] [CrossRef]
- Radulović, N.; Lazarević, J.; Ristić, N.; Palić, R. Chemotaxonomic significance of the volatiles in the genus Stachys (Lamiaceae): Essential oil composition of four Balkan Stachys species. Biochem. Syst. Ecol. 2007, 35, 196–208. [Google Scholar] [CrossRef]
- Bortolomeazzi, R.; Berno, P.; Pizzale, L.; Conte, L.S. Sesquiterpene, alkene, and alkane hydrocarbons in virgin olive oils of different varieties and geographical origins. J. Agric. Food Chem. 2001, 49, 3278–3283. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, M.; Sciarrone, D.; Crupi, M.L.; Costa, R.; Ragusa, S.; Dugo, G.; Mondello, L. Evaluation of the volatile and chiral composition in Pistacia lentiscus L. essential oil. Flavour Fragr. J. 2008, 23, 249–257. [Google Scholar] [CrossRef]
- Weyerstahl, P.; Marschall, H.; Thefeld, K.; Subba, G.C. Constituents of the essential oil from the rhizomes of Hedychium gardnerianum Roscoe. Flavour Fragr. J. 1998, 13, 377–388. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook, SRD 69. Available online: https://rb.gy/zy2yw9 (accessed on 20 August 2019).
- dos Santos, B.C.B.; da Silva, J.C.T.; Guerrero, P.G.; Leitão, G.G.; Barata, L.E.S. Isolation of chavibetol from essential oil of Pimenta pseudocaryophyllus leaf by high-speed counter-current chromatography. J. Chromatogr. A 2009, 1216, 4303–4306. [Google Scholar] [CrossRef] [PubMed]
- Mohottalage, S.; Tabacchi, R.; Guerin, P.M. Components from Sri Lankan Piper betle L. leaf oil and their analogues showing toxicity against the housefly, Musca domestica. Flavour Fragr. J. 2007, 22, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Saroglou, V.; Dorizas, N.; Kypriotakis, Z.; Skaltsa, H.D. Analysis of the essential oil composition of eight Anthemis species from Greece. J. Chromatogr. A 2006, 1104, 313–322. [Google Scholar] [CrossRef]
- Karak, S.; Acharya, J.; Begum, S.; Mazumdar, I.; Kundu, R.; De, B. Essential oil of Piper betle L. leaves: Chemical composition, anti-acetylcholinesterase, anti-β-glucuronidase and cytotoxic properties. J. Appl. Res. Med. Aromat. Plants 2018, 10, 85–92. [Google Scholar] [CrossRef]
- Syahidah, A.; Saad, C.R.; Hassan, M.D.; Rukayadi, Y.; Norazian, M.H.; Kamarudin, M.S. Phytochemical analysis, identification and quantification of antibacterial active compounds in betel leaves, Piper betle methanolic extract. Pak. J. Biol. Sci. 2017, 20, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Pin, K.Y.; Luqman Chuah, A.; Abdull Rashih, A.; Rasadah, M.A.; Law, C.L.; Choong, T.S.Y. Solid-liquid extraction of betel leaves (Piper betle L.). J. Food Process Eng. 2011, 34, 549–565. [Google Scholar] [CrossRef]
- Yusoff, Z.; Mahmud, Z.; Saleh, S.H.; Esa, Y.M. Study on the chemical constituents of Piper betle L. in relation to their possible insect attractant property. Malays. J. Sci. 2005, 24, 143–147. [Google Scholar]
- Jantan, I.; Ahmad, A.R.; Ahmad, A.S.; Ali, N.A.M. A comparative study of the essential oils of five Piper species from Peninsular Malaysia. Flavour Fragr. J. 1994, 9, 339–342. [Google Scholar] [CrossRef]
- Zamakshshari, N.; Ahmed, I.A.; Nasharuddin, M.N.A.; Mohd Hashim, N.; Mustafa, M.R.; Othman, R.; Noordin, M.I. Effect of extraction procedure on the yield and biological activities of hydroxychavicol from Piper betle L. leaves. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100320. [Google Scholar] [CrossRef]
- World Health Organization. Cortex Cinnamomi. In WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 1999; Volume 1, pp. 99–100. [Google Scholar]
- World Health Organization. Flos Caryophylli. In WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 2002; Volume 2, p. 48. [Google Scholar]
- World Health Organization. Folium Ocimi Sancti. In WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 2002; Volume 2, p. 209. [Google Scholar]
- Mohamad, N.N. Isolation of Chavibetol and Hydroxychavicol and Selected Anti-obesity Studies of Standardized Piper betle Linn. Leaf Extracts. Master’s Thesis, Universiti Sains Malaysia, Pulau Pinang, Malaysia, September 2016. [Google Scholar]
- Bajpai, V.; Sharma, D.; Kumar, B.; Madhusudanan, K.P. Profiling of Piper betle Linn. cultivars by direct analysis in real time mass spectrometric technique. Biomed. Chromatogr. 2010, 24, 1283–1286. [Google Scholar] [CrossRef]
- Adib, A.M.; Abdullah, F.; Kasim, N.; Kiong, L.S.; Ismail, N.H. 1H NMR Profiling of Piper sarmentosum Leaves: Optimisation for Sample Preparation (poster presentation). In Book of Abstracts Asian Symposium on Medicinal Plants and Spices XVII (2021)-Virtual Conference, Malaysia, 18–19 August; Ab. Ghani, N., Rasol, N.E., Eds.; Malaysian Natural Products Society (MNPS): Puncak Alam, Selangor, Malaysia, 2021. [Google Scholar]
- Contreras-Moreno, B.Z. Chemical composition of essential oil of genus Pimenta (Myrtaceae): Review. In Potential of Essential Oils; El-Shemy, H., Ed.; IntechOpen Limited: London, UK, 2018; pp. 21–39. [Google Scholar] [CrossRef] [Green Version]
- Marques, F.A.; Wendler, E.P.; Baroni, A.C.M.; De Oliveira, P.R.; Sasaki, B.S.; Guerrero, P.G. Leaf essential oil compositon of Pimenta pseudocaryophyllus (Gomes) L. R. Landrum native from Brazil. J. Essent. Oil Res. 2010, 22, 150–152. [Google Scholar] [CrossRef]
- Niculau, E.d.S.; Ribeiro, L.d.P.; Ansante, T.F.; Fernandes, J.B.; Forim, M.R.; Vieira, P.C.; Vendramim, J.D.; Da Silva, M.F.d.G.F. Isolation of chavibetol and methyl eugenol from essential oil of Pimenta pseudocaryophyllus by high performance liquid chromatography. Molecules 2018, 23, 2909. [Google Scholar] [CrossRef] [Green Version]
- de Souza, L.P.; Garbowicz, K.; Brotman, Y.; Tohge, T.; Fernie, A.R. The acetate pathway supports flavonoid and lipid biosynthesis in Arabidopsis. Plant Physiol. 2020, 182, 857–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Yang, H.; Pangestu, F.; Nikolau, B.J. Failure to maintain acetate homeostasis by acetate-activating enzymes impacts plant development. Plant Physiol. 2020, 182, 1256–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanubol, A.; Chaveerach, A.; Sudmoon, R.; Tanee, T.; Liehr, T. Verification of selected Piper species (Piperaceae) using morphological characters, molecular data, and chemical constituents. Malay. Nat. J. 2014, 66, 60–81. Available online: https://rb.gy/r00c7r (accessed on 7 November 2020).
- Verma, A.; Kumar, N.; Ranade, S.A. Genetic diversity amongst landraces of a dioecious vegetatively propagated plant, betel vine (Piper betle L.). J. Biosci. 2004, 29, 319–328. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based metabolomic analysis of plants. Nat. Protoc. 2010, 5, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.-J.; Hyun, S.-H.; Lee, S.-G.; Chun, Y.-J.; Sung, G.-H.; Choi, H.K. NMR and GC-MS based metabolic profiling and free-radical scavenging activities of Cordyceps pruinosa mycelia cultivated under different media and light conditions. PLoS ONE 2014, 9, e90823. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Zhu, Y.; Chen, X.; Liao, B.; Yao, H.; Song, J.; Chen, S.; Meng, F. The short ITS2 sequence serves as an efficient taxonomic sequence tag in comparison with the full-length ITS. BioMed Res. Int. 2013, 2013, 741476. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Yao, H.; Han, J.; Liu, C.; Song, J.; Shi, L.; Zhu, Y.; Ma, X.; Gao, T.; Pang, X.; et al. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS ONE 2010, 5, e8613. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Papadatou, M.; Argyropoulou, C.; Grigoriadou, C.; Maloupa, E.; Skaltsa, H. Essential oil content of cultivated Satureja spp. in Northern Greece. Nat. Vol. Essent. Oils 2015, 2, 37–48. [Google Scholar]
- Van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Bicchi, C.; Chaintreau, A.; Joulain, D. Identification of flavour and fragrance constituents. Flavour Fragr. J. 2018, 33, 201–202. [Google Scholar] [CrossRef] [Green Version]
- Jumhawan, U.; Putri, S.P.; Yusianto, Y.; Marwani, E.; Bamba, T.; Fukusaki, E. Selection of discriminant markers for authentication of asian palm civet coffee (Kopi luwak): A metabolomics approach. J. Agric. Food Chem. 2013, 61, 7994–8001. [Google Scholar] [CrossRef] [PubMed]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Broadhurst, D.; Goodacre, R.; Reinke, S.N.; Kuligowski, J.; Wilson, I.D.; Lewis, M.R.; Dunn, W.B. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics 2018, 14, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stettin, D.; Poulin, R.X.; Pohnert, G. Metabolomics benefits from orbitrap GC–MS—Comparison of low- and high-resolution GC–MS. Metabolites 2020, 10, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lever, J.; Krzywinski, M.; Altman, N. Points of significance: Principal component analysis. Nat. Methods 2017, 14, 641–642. [Google Scholar] [CrossRef] [Green Version]
- Bro, R.; Kjeldahl, K.; Smilde, A.K.; Kiers, H.A.L. Cross-validation of component models: A critical look at current methods. Anal. Bioanal. Chem. 2008, 390, 1241–1251. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef] [Green Version]




| Sample | Voucher Specimen No. | Coordinates | Altitude (m) |
|---|---|---|---|
| “sirih merah” | UKMB404018 | 2°59′21.4″ N, 101°42′30.3″ E | 50 |
| “sirih india” | UKMB404019 | 2°59′19.4″ N, 101°42′30.0″ E | 51 |
| “sirih melayu” | UKMB404020 | 2°59′19.6″ N, 101°42′29.7″ E | 49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, M.F.; Lee, S.Y.; Sarbini, S.R.; Mohd Faudzi, S.M.; Khamis, S.; Zainudin, B.H.; Shaari, K. Metabolomics-Driven Discovery of an Introduced Species and Two Malaysian Piper betle L. Variants. Plants 2021, 10, 2510. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10112510
Osman MF, Lee SY, Sarbini SR, Mohd Faudzi SM, Khamis S, Zainudin BH, Shaari K. Metabolomics-Driven Discovery of an Introduced Species and Two Malaysian Piper betle L. Variants. Plants. 2021; 10(11):2510. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10112510
Chicago/Turabian StyleOsman, Muhamad Faris, Soo Yee Lee, Shahrul Razid Sarbini, Siti Munirah Mohd Faudzi, Shamsul Khamis, Badrul Hisyam Zainudin, and Khozirah Shaari. 2021. "Metabolomics-Driven Discovery of an Introduced Species and Two Malaysian Piper betle L. Variants" Plants 10, no. 11: 2510. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10112510