Root Morphology, Allometric Relations and Rhizosheath of Ancient and Modern Tetraploid Wheats (Triticum durum Desf.) in Response to Inoculation with Trichoderma harzianum T-22
Abstract
:1. Introduction
2. Results
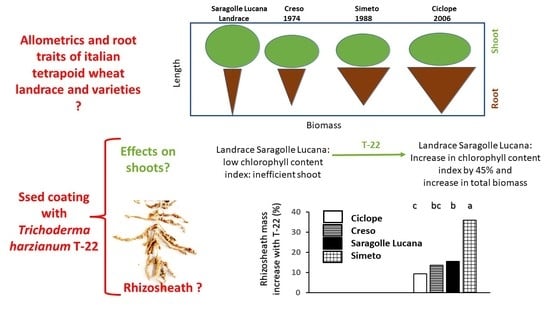
2.1. Overall Growth
2.2. Root Biometrics
2.3. Allometric Relations
2.4. Rhizosheath
3. Discussion
Shoot and Root Growth and Relations
4. Materials and Methods
4.1. Tetraploid Wheats
4.2. Inoculation Treatments
4.3. Seedling Growth Conditions
4.4. Measurements
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hanslin, H.M.; Bischoff, A.; Hovstad, K.A. Root growth plasticity to drought in seedlings of perennial grasses. Plant Soil 2019, 440, 551–568. [Google Scholar] [CrossRef]
- Agren, G.I.; Franklin, O. Root: Shoot ratios, optimization and nitrogen productivity. Ann. Bot. 2003, 92, 795–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakhforoosh, A.; Grausgruber, H.; Kauland, H.P.; Bodner, G. Dissection of drought response of modern and underutilized wheat varieties according to Passioura’s yield-water framework. Front. Plant Sci. 2015, 6, 570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakhforoosh, A.; Bodewein, T.; Fiorani, F.; Bodner, G. Identification of water use strategies at early growth stages in durum wheat from shoot phenotyping and physiological measurements. Front. Plant Sci. 2016, 7, 1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, R.A. Genetic Opportunities to Improve Cereal Root Systems for Dryland Agriculture. Plant Prod. Sci. 2008, 11, 12–16. [Google Scholar] [CrossRef]
- Wasson, A.P.; Richards, R.A.; Chatrath, R.; Misra, S.C.; Prasad, S.V.S.; Rebetzke, G.J.; Kirkegaard, J.A.; Christopher, J.; Watt, M. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J. Exp. Bot. 2012, 63, 3485–3498. [Google Scholar] [CrossRef] [Green Version]
- Motzo, R.; Atene, G.; Deidda, M. Genotypic variation in durum wheat root systems at different stages of development in a Mediterranean environment. Euphytica 1992, 66, 197–206. [Google Scholar] [CrossRef]
- Rich, S.M.; Christopher, J.; Richards, R.; Watt, M. Root phenotypes of young wheat plants grown in controlled environments show inconsistent correlation with mature root traits in the field. J. Exp. Bot. 2020, 71, 4751–4762. [Google Scholar] [CrossRef]
- Watt, M.; Moosavi, S.; Cunningham, S.C.; Kirkegaard, J.A.; Rebetzke, G.J.; Richards, R.A. A rapid, controlled-environment seedling root screen for wheat correlates well with rooting depths at vegetative, but not reproductive, stages at two field sites. Ann. Bot. 2020, 112, 447–455. [Google Scholar] [CrossRef] [Green Version]
- Figueroa-Bustos, V.; Palta, J.A.; Chen, Y.; Siddique, K.H.M. Characterization of Root and Shoot Traits in Wheat Cultivars with Putative Differences in Root System Size. Agronomy 2018, 8, 109. [Google Scholar] [CrossRef] [Green Version]
- Nakhforoosh, A.; Grausgruber, H.; Kaul, H.P.; Bodner, G. Wheat root diversity and root functional characterization. Plant Soil 2014, 380, 211–229. [Google Scholar] [CrossRef]
- Akman, H.; Akgun, N.; Tamkoc, A. Screening for root and shoot traits in different wheat species and wild wheat relatives. Bot. Sci. 2017, 95, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Gioia, T.; Nagel, K.A.; Beleggia, R.; Fragasso, M.; Ficco, D.B.; Pieruschka, R.; Vita, P.D.; Fiorani, F.; Papa, R. Impact of domestication on the phenotypic architecture of durum wheat under contrasting nitrogen fertilization. J. Exp. Bot. 2015, 66, 5519–5530. [Google Scholar] [CrossRef] [Green Version]
- Iannucci, A.; Fragasso, M.; Beleggia, R.; Nigro, F.; Papa, R. Evolution of the Crop Rhizosphere: Impact of Domestication on Root Exudates in Tetraploid Wheat (Triticum turgidum L.). Front. Plant Sci. 2017, 8, 2124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levitt, J. Responses of Plants to Environmental Stresses; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Iannucci, A.; Canfora, L.; Nigro, F.; De Vita, P.; Beleggia, R. Relationships between root morphology, root exudate compounds and rhizosphere microbial community in durum wheat. Appl. Soil Ecol. 2021, 158, 103781. [Google Scholar] [CrossRef]
- Pang, J.; Ryan, M.H.; Siddique, K.H.M.; Simpson, R.J. Unwrapping the rhizosheath. Plant Soil. 2017, 418, 129–139. [Google Scholar] [CrossRef]
- Ahmadi, K.; Zarebanadkouki, M.; Ahmed, M.A.; Ferrarini, A.; Kuzyakov, Y.; Kostka, S.I.; Carminati, A. Rhizosphere engineering: Innovative improvement of root environment. Rhizosphere 2017, 3, 176–184. [Google Scholar] [CrossRef]
- Basirat, M.; Mousavi, S.M.; Abbaszadeh, S.A.; Ebrahimi, M.; Zarebanadkouki, M. The rhizosheath: A potential root trait helping plants to tolerate drought stress. Plant Soil 2019, 445, 565–575. [Google Scholar] [CrossRef]
- Amato, M.; Bochicchio, R.; Mele, G.; Labella, R.; Rossi, R. Soil structure and stability in the spermosphere of myxosdiaspore chia (Salvia hispanica L.). Soil Res. 2018, 57, 546–558. [Google Scholar] [CrossRef]
- Di Marsico, A.; Scrano, L.; Amato, M.; Gàmiz, B.; Real, M.; Cox, L. Influence of mucilage from seeds of chia (Salvia hispanica L.) as an amendment, on the sorption-desorption of herbicides in agricultural soils. Sci. Tot. Env. 2018, 625, 531–538. [Google Scholar] [CrossRef]
- Watt, M.; Mc Cully, M.E.; Canny, M.J. Formation and stabilization of rhizosheaths of Zea mays L. Plant Physiol. 1994, 106, 179–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartnett, D.C.; Wilson, G.W.; Ott, J.; Setshogo, M. Variation in root system traits among african semi-arid savanna grasses: Implications for drought tolerance. Austral. Ecol. 2013, 38, 383–392. [Google Scholar] [CrossRef]
- Brown, L.K.; George, T.S.; Neugebauer, K.; White, P.J. The rhizosheath—A potential trait for future agricultural sustainability occurs in orders throughout the angiosperms. Plant Soil 2017, 418, 115–128. [Google Scholar] [CrossRef]
- Ndour, P.M.S.; Barry, C.M.; Tine, D.; de la Fuente Cantó, C.; Gueye, M.; Barakat, M.; Ortet, P.; Achouak, W.; Ndoye, I.; Sine, B.; et al. Pearl millet genotype impacts micro-bial diversity and enzymatic activities in relation to root-adhering soil aggregation. Plant Soil 2021, 464, 109–129. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar]
- Woo, S.L.; Lorito, M. Exploiting the interactions between fungal antagonists, pathogens and the plant for biocontrol. In Novel Biotechnologies for Biocontrol Agent Enhancement and Management; Vurro, M., Gressel, J., Eds.; NATO Security through Science Series; Springer: Dordrecht, The Netherlands, 2006; pp. 107–130. [Google Scholar]
- Vitti, A.; La Monaca, E.; Sofo, A.; Scopa, A.; Cuypers, A.; Nuzzaci, M. Beneficial effects of Trichoderma harzianum T-22 in tomato seedlings infected by Cucumber mosaic virus (CMV). Biocontrol 2015, 60, 135–147. [Google Scholar] [CrossRef]
- Vitti, A.; Pellegrini, E.; Nali, C.; Lovelli, S.; Sofo, A.; Valerio, M.; Scopa, A.; Nuzzaci, M. Trichoderma harzianum T-22 induces systemic resistance in tomato infected by Cucumber Mosaic Virus. Front. Plant Sci. 2016, 7, 1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harman, G.; Khadka, R.; Doni, F.; Uphoff, N. Benefits to plant health and productivity from enhancing plant microbial symbionts. Front. Plant Sci. 2021, 11, 610065. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based products and their widespread use in agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef] [Green Version]
- Vitti, A.; Sofo, A.; Scopa, A.; Nuzzaci, M. Sustainable agricultural practices in disease defence of traditional crops in Southern Italy: The case study of tomato cherry protected by Trichoderma harzianum T-22 Against Cucumber Mosaic Virus (CMV). In The Sustainability of Agro-Food and Natural Resource Systems in the Mediterranean Basin; Vastola, A., Ed.; Springer: Heidelberg, Germany, 2015; pp. 133–143. [Google Scholar]
- Sarrocco, S.; Vannacci, G. Preharvest application of beneficial fungi as a strategy to prevent postharvest mycotoxin contamination: A review. Crop. Prot. 2018, 110, 160–170. [Google Scholar] [CrossRef]
- Kthiri, Z.; Jabeur, M.B.; Machraoui, M.; Gargouri, S.; Hiba, K.; Hamada, W. Coating seeds with Trichoderma strains promotes plant growth and enhance the systemic resistance against Fusarium crown rot in durum wheat. Egypt J. Biol. Pest. Control 2020, 30, 1–10. [Google Scholar] [CrossRef]
- Lucini, L.; Colla, G.; Miras Moreno, M.B.; Bernardo, L.; Cardarelli, M.; Terzi, V.; Bonini, P.; Rouphaelf, Y. Inoculation of Rhizoglomus irregulare or Trichoderma atroviride differentially modulates metabolite profiling of wheat root exudates. Phytochemistry 2019, 157, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield and nutritional quality of leafy vegetables. Fron. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucci, M.; Ruocco, M.; De Masi, L.; De Palma, M.; Lorito, M. The beneficial effect of Trichoderma spp. on tomato is modulated by the plant genotype. Mol. Plant Pathol. 2011, 12, 341–354. [Google Scholar] [PubMed]
- Sofo, A.; Scopa, A.; Manfra, M.; De Nisco, M.; Tenore, G.; Troisi, J.; Di Fiori, R.; Novellino, E. Trichoderma harzianum strain T-22 induces changes in phytohormone levels in cherry rootstocks (Prunus cerasus × P. canescens). Plant Growth Regul. 2011, 65, 421–425. [Google Scholar] [CrossRef] [Green Version]
- Sofo, A.; Tataranni, G.; Xiloyannis, C.; Dichio, B.; Scopa, A. Direct effects of Trichoderma harzianum strain T-22 on micropropagated GiSeLa6® (Prunus cerasus × Prunus canescens) rootstocks. Environ. Exp. Bot. 2012, 76, 33–38. [Google Scholar] [CrossRef]
- Illescas, M.; Pedrero-Méndez, A.; Pitorini-Bovolini, M.; Hermosa, R.; Monte, E. Phytohormone Production Profiles in Trichoderma Species and Their Relationship to Wheat Plant Responses to Water Stress. Pathogens 2021, 10, 991. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macias-Rodriguez, L.; Cortes-Penagos, C.; Lopez-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras-Cornejo, H.A.; Macias-Rodriguez, L.; Alfaro-Cuevas, R.; Lopez-Bucio, J. Trichoderma spp. improve growth of Arabidopsis seedlings under salt stress through enhanced root development, osmolyte production, and Na+ elimination through root exudates. Mol. Plant-Microbe Interact. 2014, 27, 503–514. [Google Scholar] [CrossRef] [Green Version]
- López-Coria, M.; Hernández-Mendoza, J.L.; Sánchez-Nieto, S. Trichoderma asperellum Induces Maize Seedling Growth by Activating the Plasma Membrane H+-ATPase. Mol. Plant Microbe Interact. 2016, 29, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Brouwer, R. Nutritive influences on the distribution of dry matter in the plant. Neth. J. Agric. Sci. 1962, 10, 399–408. [Google Scholar] [CrossRef]
- Davidson, R.L. Effect of root/leaf temperature differentials on root/shoot ratios in some pasture grasses and clover. Ann. Bot. 1969, 33, 561–569. [Google Scholar] [CrossRef]
- Wojciechowski, T.; Gooding, M.J.; Ramsay, L.; Gregory, P.J. The effects of dwarfing genes on seedling root growth of Wheat. J. Exp. Bot. 2009, 60, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Nakhforoosh, A.; Nagel, K.A.; Fiorani, F.; Bodner, G. Deep soil exploration vs. topsoil exploitation: Distinctive rooting strategies between wheat landraces and wild relatives. Plant Soil 2021, 459, 397–421. [Google Scholar] [CrossRef] [PubMed]
- Bakhshandeh, S.; Kertesz, M.A.; Corneo, P.E.; Dijkstra, F.A. Dual-labeling with 15N and H218O to investigate water and N uptake of wheat under different water regimes. Plant Soil 2016, 408, 429–441. [Google Scholar] [CrossRef]
- Valente, F.G.; Le Gouis, J.; Moënne-Loccoz, Y.; Prigent–Combaret, C. Ancient wheat varieties have a higher ability to interact with plant growth-promoting rhizobacteria. Plant Cell Environ. 2019, 43, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Haling, R.E.; Brown, L.K.; Bengough, A.G.; Young, I.M.; Hallett, P.D.; White, P.J.; George, T.S. Root hairs improve root penetration, root–soil contact, and phosphorus acquisition in soils of different strength. J. Exp. Bot 2013, 64, 3711–3721. [Google Scholar] [CrossRef] [Green Version]
- Marzario, S.; Logozzo, G.; David, J.L.; Zeuli, P.S.; Gioia, T. Molecular Genotyping (SSR) and Agronomic Phenotyping for Utilization of Durum Wheat (Triticum durum Desf.) Ex Situ Collection from Southern Italy: A Combined Approach Including Pedigreed Varieties. Genes 2018, 9, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangini, G.; Gadaleta, A.; Colasuonno, P.; Marcotuli, I.; Signorile, A.M.; Simeone, R.; Vita, P.D.; Mastrangelo, A.M.; Laidò, G.; Pecchioni, N.; et al. Genetic dissection of the relationships between grain yield components by genome-wide association mapping in a collection of tetraploid wheats. PLoS ONE 2018, 13, e0190162. [Google Scholar]
- Kthiri, Z.; Jabeur, M.B.; Chairi, F.; López-Cristoffanini, C.; López-Carbonell, M.; Serret, M.D.; Araus, J.L.; Karmous, C.; Hamada, W. Exploring the Potential of Meyerozyma guilliermondii on Physiological Performances and Defense Response against Fusarium Crown Rot on Durum Wheat. Pathogens 2021, 10, 52. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bochicchio, R.; Labella, R.; Vitti, A.; Nuzzaci, M.; Logozzo, G.; Amato, M. Root Morphology, Allometric Relations and Rhizosheath of Ancient and Modern Tetraploid Wheats (Triticum durum Desf.) in Response to Inoculation with Trichoderma harzianum T-22. Plants 2022, 11, 159. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11020159
Bochicchio R, Labella R, Vitti A, Nuzzaci M, Logozzo G, Amato M. Root Morphology, Allometric Relations and Rhizosheath of Ancient and Modern Tetraploid Wheats (Triticum durum Desf.) in Response to Inoculation with Trichoderma harzianum T-22. Plants. 2022; 11(2):159. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11020159
Chicago/Turabian StyleBochicchio, Rocco, Rosanna Labella, Antonella Vitti, Maria Nuzzaci, Giuseppina Logozzo, and Mariana Amato. 2022. "Root Morphology, Allometric Relations and Rhizosheath of Ancient and Modern Tetraploid Wheats (Triticum durum Desf.) in Response to Inoculation with Trichoderma harzianum T-22" Plants 11, no. 2: 159. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11020159