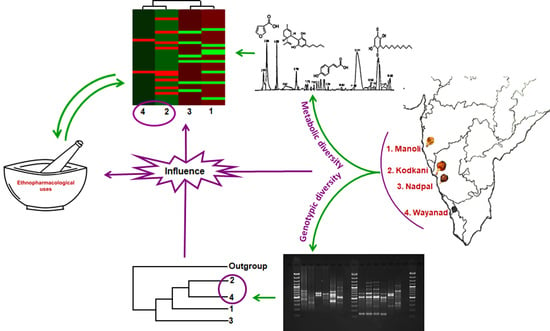
Assessing the Impact of Geographical Distribution and Genetic Diversity on Metabolic Profiles of a Medicinal Plant, Embelia ribes Burm. f.
Abstract
:1. Introduction
2. Results
2.1. Molecular Identification of Fruit Samples Collected from Different Geographical Regions
2.2. Metabolic Profiles of Embelia Fruits Vary across the Accessions
2.3. Identified Compounds Represent Major Chemical Groups and Potential Medicinal Properties
2.4. Accessions of Embelia Are Genetically Diverse
2.5. Metabolic Diversity in Embelia Fruits Correlates with Genotypic Diversity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Metabolite Extraction and LC-MS/MS Acquisition
4.3. LC-MS/MS Data Analysis
4.4. Genomic DNA Isolation, and Polymerase Chain Reaction (PCR)
4.5. Sequencing and Data Analysis
4.6. Analysis of Genetic Diversity and Construction of Phylogenetic Tree
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burman, N.L. Nicolai Laurentii Burmanni Flora Indica; Haek: Amsterdam, The Netherlands, 1768. [Google Scholar]
- Lal, B.; Mishra, N. Importance of Embelia ribes: An update. Int. J. Pharm. Sci. Res. 2013, 4, 3823. [Google Scholar]
- Udayan, P.; Harinarayanan, M.; Tushar, K.; Balachandran, I. Some Common Plants Used by Kurichiar Tribes of Tirunelli Forest, Wayanad District, Kerala in Medicine and Other Traditional Uses. Ind. J. Tradit. Knowl. 2008, 7, 250–255. [Google Scholar]
- Thyloor, R. Phytochemical analysis of Embelia ribes seeds for antimicrobial activities. J. Med. Plants 2018, 6, 41–43. [Google Scholar]
- Chopda, M.; Mahajan, R. Wound healing plants of Jalgaon district of Maharashtra state, India. Ethnobot. Leafl. 2009, 2009, 1. [Google Scholar]
- Albert, A.; Sareedenchai, V.; Heller, W.; Seidlitz, H.K.; Zidorn, C. Temperature is the key to altitudinal variation of phenolics in Arnica montana L. cv. ARBO. Oecologia 2009, 160, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Krahmer, A.; Herwig, N.; Hadian, J.; Schulz, H.; Meiners, T. Metabolomics approaches for analyzing effects of geographic and environmental factors on the variation of root essential oils of Ferula assa-foetida L. J. Agric. Food Chem. 2020, 68, 9940–9952. [Google Scholar] [CrossRef]
- Saurabh, K.B. Total phenolic content and antioxidant activity of extracts of Bridelia retusa Spreng Bark: Impact of dielectric constant and geographical location. J. Med. Plants Res. 2011, 5, 817–822. [Google Scholar]
- Namdeo, A.G.; Sharma, A.; Fulzele, D.P.; Mahadik, K.R. Influence of geographical and climatic conditions on camptothecin content of Nothapodytes nimmoniana. Rec. Nat. Prod. 2010, 4, 64. [Google Scholar]
- Kamble, V.; Attar, U.; Umdale, S.; Nimbalkar, M.; Ghane, S.; Gaikwad, N. Phytochemical analysis, antioxidant activities and optimized extraction of embelin from different genotypes of Embelia ribes Burm f.: A woody medicinal climber from Western Ghats of India. Physiol. Mol. Biol. Plants 2020, 26, 1855–1865. [Google Scholar] [CrossRef]
- Atlabachew, M.; Mehari, B.; Combrinck, S.; McCrindle, R. Single-step isolation of embelin using high-performance countercurrent chromatography and determination of the fatty acid composition of seeds of Embelia schimperi. Biomed. Chromatogr. 2017, 31, e4018. [Google Scholar] [CrossRef]
- Kamble, G.S.; Torane, R.C.; Ghayal, N.A.; Tambe, A.S.; Deshpande, N.R.; Salvekar, J.P. GC-MS study of fatty acids and esters from Embelia basal. J. Pharm. Res. 2010, 3, 2608–2609. [Google Scholar]
- Puiggròs, F.; Solà, R.; Bladé, C.; Salvadó, M.-J.; Arola, L. Nutritional biomarkers and foodomic methodologies for qualitative and quantitative analysis of bioactive ingredients in dietary intervention studies. J. Chromatogr. A 2011, 1218, 7399–7414. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, C.; Valdés, A.; García-Cañas, V.; Simó, C.; Celebier, M.; Rocamora-Reverte, L.; Gómez-Martínez, Á.; Herrero, M.; Castro-Puyana, M.; Segura-Carretero, A. Global Foodomics strategy to investigate the health benefits of dietary constituents. J. Chromatogr. A 2012, 1248, 139–153. [Google Scholar] [CrossRef]
- Hoffmann, J.F.; Carvalho, I.R.; Barbieri, R.L.; Rombaldi, C.V.; Chaves, F.C. Butia spp. (Arecaceae) LC-MS-based metabolomics for species and geographical origin discrimination. J. Agric. Food Chem. 2017, 65, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Brinckmann, J.A. Geographical indications for medicinal plants: Globalization, climate change, quality and market implications for geo-authentic botanicals. World J. Tradit. Chin. Med. 2015, 1, 16. [Google Scholar] [CrossRef]
- Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: A model for environmental metabolomics of plants. Sci. Rep. 2016, 6, 1–11. [Google Scholar]
- Chrungoo, N.; Rout, G.; Balasubramani, S.; Rajasekharan, P.; Haridasan, K.; Rao, B.; Manjunath, R.; Nagduwar, G.; Venkatasubramanian, P.; Nongbet, A. Establishing taxonomic identity and selecting genetically diverse populations for conservation of threatened plants using molecular markers. Curr. Sci. 2018, 114, 539–553. [Google Scholar] [CrossRef]
- Dwivedi, S.; Ghatuary, S.K.; Prasad, S.; Jain, P.K.; Parkhe, G. Phytochemical Screening and In Vivo Anti-inflammatory Activity of Hydroalcoholic Extract of Embelia Ribes Burm. F. J. Drug Deliv. Ther. 2019, 9, 386–389. [Google Scholar]
- Saraf, A.; Srinivas, K.S.; Chaturvedi, A. Phytochemical and Elemental Profile of Embelia ribes Burn. F. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 471–476. [Google Scholar]
- Jelinski, D.E. On genes and geography: A landscape perspective on genetic variation in natural plant populations. Landsc. Urban Plan. 1997, 39, 11–23. [Google Scholar] [CrossRef]
- Gitzendanner, M.A.; Soltis, P.S. Patterns of genetic variation in rare and widespread plant congeners. Am. J. Bot. 2000, 87, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Byrne, M.; Coates, D.; Macdonald, B.; McArthur, S.; Van Leeuwen, S. Contrasting influences of geographic range and distribution of populations on patterns of genetic diversity in two sympatric Pilbara acacias. PLoS ONE 2016, 11, e0163995. [Google Scholar] [CrossRef] [Green Version]
- Szczecińska, M.; Sramko, G.; Wołosz, K.; Sawicki, J. Genetic diversity and population structure of the rare and endangered plant species Pulsatilla patens (L.) Mill in East Central Europe. PLoS ONE 2016, 11, e0151730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasi, R.; Rajendran, A. Diversity of wild fruits in Nilgiri Hills of the Southern Western Ghats-ethnobotanical aspects. Int. J. Appl. Biol. Pharm. Technol 2012, 3, 82–87. [Google Scholar]
- Oritani, Y.; Okitsu, T.; Nishimura, E.; Sai, M.; Ito, T.; Takeuchi, S. Enhanced glucose tolerance by intravascularly administered piceatannol in freely moving healthy rats. Biochem. Biophys. Res. Commun. 2016, 470, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.J.; Rimando, A.M.; Mizuno, C.S.; Mathews, S.T. α-Glucosidase inhibitory effect of resveratrol and piceatannol. J. Nutr. Biochem. 2017, 47, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sanyal, S.; Kanwar, U. Antispermatogenic effect of embelin, a plant benzoquinone, on male albino rats in vivo and in vitro. Contraception 1989, 39, 307–320. [Google Scholar] [CrossRef]
- Ogbuewu, I.P.; Unamba-Oparah, I.C.; Odoemenam, V.U.; Etuk, I.F.; Okoli, I.C. The potentiality of medicinal plants as the source of new contraceptive principles in males. North Am. J. Med. Sci. 2011, 3, 255. [Google Scholar] [CrossRef]
- Etta, H.E.; Bassey, U.P.; Eneobong, E.E.; Okon, O.B. Anti-spermatogenic effects of ethanol extract of Mucuna urens. J. Reprod. Contracept. 2009, 20, 161–168. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Khan, S.; Qureshi, M.I.; Alam, T.; Abdin, M. Protocol for isolation of genomic DNA from dry and fresh roots of medicinal plants suitable for RAPD and restriction digestion. Afr. J. Biotechnol. 2007, 6, 175. [Google Scholar]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Perrier, X.; Jacquemoud-Collet, J.-P. DARwin software. 2006. 2015. Available online: https://darwin.cirad.fr/ (accessed on 30 November 2021).





| Sr. No. | Molecular Formula | Abundance (%) | Adducts | Mass | m/z | RT | Score | Compound Name | M | K | N | W |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | C6 H4O2 | 167.0353 (100), 108.0219 (84.96), 153.0198 (16.49), 123.0113 (0.46) | (M+HCOO-), (M+CH3COO-) | 108.0211 | 167.0556 | 9.141 | 98.87 | 1,2-Benzoquinone | - | + | - | + |
| 2 | C5H12S2 | 195.0514 (100), 129.0197 (57.31), 177.0409 (6.16), 159.0312 (7.27) | (M-H-), (M+CH3COO-) | 136.0387 | 135.0307 | 2.922 | 81.86 | 1-pentanesulfenothoic acid | - | + | - | + |
| 3 | C6H8O5 | 111.0092 (100), 125.0248 (11.05), 143.0351 (7.81), 169.0146 (23.32) | (M-H-), (M+CH3COO-), (M+HCOO-) | 160.0372 | 205.0361 | 6.443 | 97.15 | 2-Formylglutarate | - | + | - | + |
| 4 | C15H12O5 | 271.061 (100), 151.0035 (32.56), 119.051 (13.26), 107.0127 (2.66), 177.0177 (4.73) | (M-H-), (M+CH3COO-), (M+HCOO-) | 272.0685 | 271.0626 | 10.89 | 93.68 | 3,4,5-Trihydroxyflavanone | - | + | - | - |
| 5 | C4H8O4 | 136.8745 (100), 134.8767 (72.48), 119.3337 (30.23), 138.8695 (33.82), 112.4422 (23.18) | (M-H-), (M+CH3COO-), (M+HCOO-) | 120.0423 | 179.0566 | 3.756 | 94.87 | 3,4-Dihydroxybutyric acid | - | + | - | + |
| 6 | C9H11NO3 | 163.0407 (100), 180.068 (82.43),135.0464 (91.86), 119.0486 (34.69), 121.0316 (3.34) | (M-H-), (M+CH3COO-) | 181.0739 | 180.0672 | 4.68 | 84.77 | 3-Amino-3-(4-hydroxyphenyl) propanoate | - | + | - | + |
| 7 | C5H4O3 | 111.0091 (100), 112.9853 (59.93), 105.6042 (10.74), 137.3377 (5.76), 168.1806 (11.1) | (M-H-), (M+CH3COO-) | 112.016 | 111.0093 | 3.256 | 85.76 | 3-Furoic acid | - | + | - | + |
| 8 | C7H12O5 | 119.0352 (100), 163.0618 (18.61), 101.0256 (5.33), 221.0677 (16.91) | (M-H-), (M+CH3COO-) | 176.0685 | 221.0675 | 6.781 | 93.91 | 3-Isopropylmalic acid | + | + | - | + |
| 9 | C6H6O6 | 111.0089 (100), 101.049 (0.93), 117.0174 (4.29), 129.0177 (1.58), 155.0332 (5.15), 173.0828 (2.73), 175.0604 (12.66), 191.02185(0.34) | (M-H-), | 174.0164 | 173.0097 | 6.363 | 97.02 | Aconitic acid | - | + | - | + |
| 10 | C7H6O2 | 121.0298 (100), 120.0214 (20.48), 119.9136 (0.35), 109.8534 (0.28) | (M-H-), (M+CH3COO-) | 122.0368 | 121.0356 | 9.141 | 96.01 | Benzoic acid | - | + | - | + |
| 11 | C28H40O4 | 439.2885 (100), 279.2339 (8.98), 101.0243 (2.78), 170.8337 (1.48), 131.0358 (0.79), 161.0442 (0.84) | (M-H-), (M+CH3COO-) | 440.2954 | 439.2854 | 15.52 | 86.76 | Bolegrevilol | - | - | - | + |
| 12 | C6H8O7 | 111.0093 (100), 129.0197 (5.64), 131.9995 (0.18), 154.9984 (0.71), 173.0095 (1.3) | (M-H-), (M+CH3COO-) | 192.027 | 191.0201 | 4.259 | 95.64 | Citric acid | + | + | - | + |
| 13 | C9H8O3 | 119.0509 (100), 163.0407 (8.71), 117.9266 (0.38), 121.0308 (2.97) | (M-H-), (M+CH3COO-) | 164.0484 | 163.0412 | 8.88 | 93.57 | Coumaric acid | - | + | - | - |
| 14 | C10H18O4 | 201.1134 (100), 183.1021 (59.57), 139.7911 (65.43), 140.1177 (13.75), 141.11 (5.96), 157.0852 (5.47) | (M-H-) | 202.1205 | 201.1143 | 9.982 | 95.36 | Decanedioic acid | + | + | - | + |
| 15 | C13H24O2 | 213.1868 (100), 195.1759 (2.92), 193. 1605 (0.16), 211.3199 (0.06), 257.1764 (0.46), 196.179 (0.83) | (M-H-), (M+CH3COO-) | 212.1776 | 271.1918 | 15.268 | 85.1 | Delta-tridecalactone | + | + | - | + |
| 16 | C6H10S2 | 111.0093 (100), 191.0208 (10.89), 173.9144 (0.08), 154.9984 (0.71), 147.0297 (0.9), 130.9991 (0.88) | (M-H-) | 146.0224 | 191.0199 | 4.26 | 81.97 | Di-2-propenyl disulfide | - | + | + | + |
| 17 | C8H14O2S2 | 111.0095 (100), 125.0248 (12.2), 169.0149 (30.75), 101.0249 (0.1), 143.0356 (6.94), 154.9997 (2.13), 205.0366 (2.19), 173.0102 (1.98) | (M-H-), (M+CH3COO-) | 206.0435 | 205.0365 | 6.365 | 80.91 | DL-alpha-Lipoic acid | - | + | - | + |
| 18 | C17H26O4 | 293.1776 (100), 265.1822 (10.08), 152.0117(23.46), 195.1762 (0.05), 223.1980 (0.02), 265.1817 (0.98), 275.1672 (0.04), 179.0336 (0.02), 151.0399 (0.73), 163.0405 (0.03), 165.0197 (0.58), 193.1603 (0.01) | (M-H-) | 294.1831 | 293.1771 | 14.42 | 94.7 | Embelin | + | + | + | + |
| 19 | C6H14S2 | 195.0514 (100), 129.0197 (63.16), 101.0232 (1.78), 100.0129 (1.67) | (M-H-), (M+CH3COO-) | 150.0537 | 195.0514 | 2.91 | 83.13 | Ethyl-1-methylpropyl disulfide | - | + | - | + |
| 20 | C6H12O7 | 195.0541 (100), 129.0197 (63.16), 111.0089 (1.93), 101.0232 (1.78), 141.0172 (1.17), 100.0129 (1.67), 151.06 (2.87), 159.02 (4.72), 177.04 (7.5) | (M+CH3COO-) | 196.0583 | 195.0514 | 2.92 | 96.12 | Galactonic acid | - | + | - | + |
| 21 | C16H30O4 | 285.2076 (100), 195.1734 (0.69), 160.314 (0.24), 151.98(0.44) | (M-H-), (M+CH3COO-) | 286.2144 | 285.2083 | 11.57 | 94.66 | Hexadecanedioic acid | - | + | - | + |
| 22 | C21H20O12 | 463.0907 (100), 301.0354 (9.29), 316.0235 (4.15), 300.2459 (7.06), 271.0264 (1.1), 178.9978 (0.59), 287.0243 (0.21) | (M-H-) | 464.0948 | 523.1085 | 8.79 | 76.8 | Isoquercitrin | - | + | - | - |
| 23 | C4H6O5 | 115.0039 (100), 133.0137 (25.99), 134.0181(0.08), 103.0701 (2.41), 105.3022 (3.11) | (M-H-) | 134.0215 | 133.0148 | 3.16 | 98.24 | Malic acid | - | + | - | + |
| 24 | C15H12O5 | 271.062 (100), 151.0037 (37.98), 119.0509 (10.92), 177.0197 (5.23), 107.0142 (4.58) | (M-H-) | 272.0697 | 271.0623 | 10.90 | 91.8 | Naringenin | - | + | - | - |
| 25 | C7H14S2 | 119.0352 (100), 163.0618 (18.61), 221.0677 (16.91), 101.0256 (5.33) | (M-H-), (M+CH3COO-) | 162.0537 | 221.0675 | 6.78 | 85.79 | N-propyl sec-butyl disulfide | + | + | + | + |
| 26 | C4H4O5 | 111.0092 (100), 103.1223 (0.39), 106.2887 (0.38), 129.01 (7.82), 191.0208 (10.89) | (M-H-), (M+CH3COO-) | 132.0059 | 191.0201 | 4.26 | 98.76 | Oxaloacetate | - | + | - | + |
| 27 | C14H12O4 | 261.0395 (100), 103.0404 (17.34), 111.0115 (25.8), 135.0417 (13.41), 137.0252 (59.38), 148.0374 (31.73), 167.0366 (27.76), 231.0277 (50.05) | (M+CH3COO-) | 244.0736 | 303.0885 | 8.37 | 94.96 | Piceatannol | - | + | - | - |
| 28 | C15H22O2 | 293. 1772 (100), 152.01 (1.17), 124.0171 (1.09), 265.1818 (0.98), 166.0278 (0.29), 275.1665 (0.04), 195.1756 (0.01) | (M+CH3COO-), (M+HCOO-) | 234.1626 | 293.1776 | 14.51 | 95.39 | Procurcumenol | - | + | + | + |
| 29 | C15H10O7 | 301.0363 (100), 107.0142 (1.63), 151.0034 (19.11), 121.0294(2.17), 178.9991(14.1) | (M-H-) | 302.0427 | 301.0367 | 10.15 | 88.89 | Quercetin | - | + | - | - |
| 30 | C19H30O4 | 321.2089 (100), 124.0169 (0.71), 152.0117 (1.01), 293.2134 (0.66), 166.0257 (0.18), 317.8458 (0.09) | (M-H-) | 322.2144 | 321.2093 | 13.16 | 92.93 | Rapanone | - | + | - | + |
| 31 | C14H28 | 255.2335 (100), 210.9515 (6.19), 134.0953 (3.02), 119.037 (4.12), 112.98 (2.76) | (M+CH3COO-) | 196.2191 | 255.2338 | 0.572 | 96.94 | Tetradecene | + | + | - | + |
| 32 | C11H22O | 229.1825 (100), 112.9864 (0.85), 151.9661 (0.95), 183.1758 (43.74) | (M+CH3COO-), (M+HCOO-) | 170.1675 | 229.1817 | 11.31 | 93.1 | Undecan-2-one | - | + | - | + |
| 33 | C8H8O4 | 167.0354 (100),108.0222 (95.03), 168.8361 (83.37), 152.0119 (14.39), 128.0691 (3.21), 125.0234 (1.95) | (M-H-) | 168.0429 | 167.0356 | 9.14 | 83.19 | Vanillic acid | - | + | - | + |
| 34 | C18H28N2O4 | 127.1205 (100), 129.1004 (58.35), 145.1369 (55.08), 319.1974 (15.5), 273.1822 (30.12), 147.1169 (30.35), 175.1199 (44.14), 301.1838 (12.14), 109.1038 (27.28) | (M+H+) | 336.2049 | 337.2135 | 3.16 | 75.88 | Acebutolol | - | + | + | - |
| 35 | C21H30O2 | 127.1204 (100), 129.1005 (55.48), 145.1367 (55), 175.1199 (46.27), 147.1168 (28.57), 109.1039 (27.64), 157.1043 (14.87), 111.084 (3.29), 193.1358 (9.45), 163.1531 (4.65), 139.0882 (11.36), 273.1821 (28.48), 301.1835 (12.39), 291.1975 (7.41), 319.1984 (15.9) | (M+Na+) | 314.2246 | 337.2134 | 4.17 | 76.21 | Cannabidiol | - | + | + | - |
| 36 | C12H20O2 | 109.1032 (100), 219.1348 (16.32), 204.1907 (16.05), 202.1745 (25.12), 187.1584 (22.13), 169.1432 (52.16), 151.1255 (9.44), 145.1362 (3.92), 111.1186 (6.99), 106.1057 (0.58), 127.1189 (29.49) | (M+Na+) | 196.1463 | 219.1353 | 2.83 | 73.22 | Hexenyl-(3z)-hexenoate (3z-) | + | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raskar, S.; Purkar, V.; Sardesai, M.; Mitra, S. Assessing the Impact of Geographical Distribution and Genetic Diversity on Metabolic Profiles of a Medicinal Plant, Embelia ribes Burm. f. Plants 2022, 11, 2861. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11212861
Raskar S, Purkar V, Sardesai M, Mitra S. Assessing the Impact of Geographical Distribution and Genetic Diversity on Metabolic Profiles of a Medicinal Plant, Embelia ribes Burm. f. Plants. 2022; 11(21):2861. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11212861
Chicago/Turabian StyleRaskar, Shubhangi, Vishwabandhu Purkar, Milind Sardesai, and Sirsha Mitra. 2022. "Assessing the Impact of Geographical Distribution and Genetic Diversity on Metabolic Profiles of a Medicinal Plant, Embelia ribes Burm. f." Plants 11, no. 21: 2861. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11212861