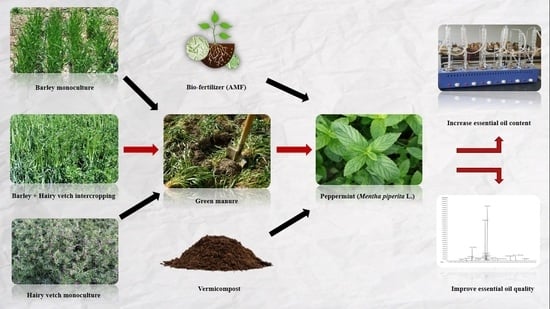
Effects of Green Manures (in the Form of Monoculture and Intercropping), Biofertilizer and Organic Manure on the Productivity and Phytochemical Properties of Peppermint (Mentha piperita L.)
Abstract
:1. Introduction
2. Results
2.1. Green Manures Productivity
2.2. Peppermint
2.2.1. Nutrient
2.2.2. Agronomic Traits
2.2.3. Leaf Greenness
2.2.4. Dry Yield
2.2.5. Essential Oil Content
2.2.6. Essential Oil Yield
2.2.7. Essential Oil Compositions
3. Discussion
4. Materials and Methods
4.1. Site Description
4.2. Experimental Design and Treatments
4.3. Measurements
4.3.1. The Fresh Biomass Yield of Green Manures
4.3.2. Peppermint
Dry Matter Yield
Leaf Greenness Index (SPAD)
Essential Oil Extraction and Analysis
Nutrient Concentration
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amani Machiani, M.; Javanmard, A.; Habibi Machiani, R.; Sadeghpour, A. Arbuscular Mycorrhizal Fungi and Changes in Primary and Secondary Metabolites. Plants 2022, 11, 2183. [Google Scholar] [CrossRef]
- Eftekhari, A.; Khusro, A.; Ahmadian, E.; Dizaj, S.M.; Dinparast, L.; Bahadori, M.B.; Hasanzadeh, A.; Cucchiarini, M. Phytochemical and Nutra-Pharmaceutical Attributes of Mentha spp.: A Comprehensive Review. Arab. J. Chem. 2021, 14, 103106. [Google Scholar] [CrossRef]
- Soltanbeigi, A.; Özgüven, M.; Hassanpouraghdam, M.B. Planting-Date and Cutting-Time Affect the Growth and Essential Oil Composition of Mentha × Piperita and Mentha arvensis. Ind. Crops Prod. 2021, 170, 113790. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Javanmard, A.; Morshedloo, M.R.; Maggi, F. Evaluation of Competition, Essential Oil Quality and Quantity of Peppermint Intercropped with Soybean. Ind. Crops Prod. 2018, 111, 743–754. [Google Scholar] [CrossRef]
- Gholamipourfard, K.; Salehi, M.; Banchio, E. Mentha Piperita Phytochemicals in Agriculture, Food Industry and Medicine: Features and Applications. S. Afr. J. Bot. 2021, 141, 183–195. [Google Scholar] [CrossRef]
- Ostadi, A.; Javanmard, A.; Amani Machiani, M.; Morshedloo, M.R.; Nouraein, M.; Rasouli, F.; Maggi, F. Effect of Different Fertilizer Sources and Harvesting Time on the Growth Characteristics, Nutrient Uptakes, Essential Oil Productivity and Composition of Mentha x Piperita L. Ind. Crops Prod. 2020, 148, 112290. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Javanmard, A.; Morshedloo, M.R.; Maggi, F. Evaluation of Yield, Essential Oil Content and Compositions of Peppermint (Mentha piperita L.) Intercropped with Faba Bean (Vicia faba L.). J. Clean. Prod. 2018, 171, 529–537. [Google Scholar] [CrossRef]
- Javanmard, A.; Ashrafi, M.; Morshedloo, M.R.; Machiani, M.A.; Rasouli, F.; Maggi, F. Optimizing Phytochemical and Physiological Characteristics of Balangu (Lallemantia iberica) by Foliar Application of Chitosan Nanoparticles and Myco-Root Inoculation under Water Supply Restrictions. Horticulturae 2022, 8, 695. [Google Scholar] [CrossRef]
- Lei, B.; Wang, J.; Yao, H. Ecological and Environmental Benefits of Planting Green Manure in Paddy Fields. Agriculture 2022, 12, 223. [Google Scholar] [CrossRef]
- Ablimit, R.; Li, W.; Zhang, J.; Gao, H.; Zhao, Y.; Cheng, M.; Meng, X.; An, L.; Chen, Y. Altering Microbial Community for Improving Soil Properties and Agricultural Sustainability during a 10-Year Maize-Green Manure Intercropping in Northwest China. J. Environ. Manag. 2022, 321, 115859. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, G.; Chang, D.; Gao, S.; Han, M.; Zhang, J.; Sun, X.; Cao, W.D. Transfer Characteristics of Nitrogen Fixed by Leguminous Green Manure Crops When Intercropped with Maize in Northwestern China. J. Integr. Agric. 2022, 21, 1177–1187. [Google Scholar] [CrossRef]
- Maitra, S.; Hossain, A.; Brestic, M.; Skalicky, M.; Ondrisik, P.; Gitari, H.; Brahmachari, K.; Shankar, T.; Bhadra, P.; Palai, J.B.; et al. Intercropping—A Low Input Agricultural Strategy for Food and Environmental Security. Agronomy 2021, 11, 343. [Google Scholar] [CrossRef]
- Hwang, H.Y.; Kim, G.W.; Lee, Y.B.; Kim, P.J.; Kim, S.Y. Improvement of the Value of Green Manure via Mixed Hairy Vetch and Barley Cultivation in Temperate Paddy Soil. Field Crop. Res. 2015, 183, 138–146. [Google Scholar] [CrossRef]
- Kumar, A.; Jnanesha, A.C.; Verma, R.K.; Kumar, D.; Lal, R.K. Phytoremediation, Eco-Restoration, and Adaptive Response of Lemongrass (C. flexuosus Wats) Grown on Fly Ash and Vermicompost Improved Quality Essential Oil Yield. Biochem. Syst. Ecol. 2022, 104, 104457. [Google Scholar] [CrossRef]
- Yilmaz, A.; Karik, Ü. AMF and PGPR Enhance Yield and Secondary Metabolite Profile of Basil (Ocimum basilicum L.). Ind. Crops Prod. 2022, 176, 114327. [Google Scholar] [CrossRef]
- Rezaei-Chiyaneh, E.; Battaglia, M.L.; Sadeghpour, A.; Shokrani, F.; Nasab, A.D.M.; Raza, M.A.; von Cossel, M. Optimizing Intercropping Systems of Black Cumin (Nigella sativa L.) and Fenugreek (Trigonella foenum-graecum L.) through Inoculation with Bacteria and Mycorrhizal Fungi. Adv. Sustain. Syst. 2021, 5, 2000269. [Google Scholar] [CrossRef]
- Johny, L.; Cahill, D.M.; Adholeya, A. AMF Enhance Secondary Metabolite Production in Ashwagandha, Licorice, and Marigold in a Fungi-Host Specific Manner. Rhizosphere 2021, 17, 100314. [Google Scholar] [CrossRef]
- Khalediyan, N.; Weisany, W.; Schenk, P.M. Arbuscular Mycorrhizae and Rhizobacteria Improve Growth, Nutritional Status and Essential Oil Production in Ocimum basilicum and Satureja hortensis. Ind. Crops Prod. 2021, 60, 113163. [Google Scholar] [CrossRef]
- Chatterjee, D.; Dutta, S.K.; Kikon, Z.J.; Kuotsu, R.; Sarkar, D.; Satapathy, B.S.; Deka, B.C. Recycling of Agricultural Wastes to Vermicomposts: Characterization and Application for Clean and Quality Production of Green Bell Pepper (Capsicum annuum L.). J. Clean. Prod. 2021, 315, 128115. [Google Scholar] [CrossRef]
- Heidarpour, O.; Esmaielpour, B.; Soltani, A.A.; Khorramdel, S. Effect of Vermicompost on Essential Oil Composition of (Satureja hortensis L.) Under Water Stress Condition. J. Essent. Oil-Bear. Plants 2019, 22, 484–492. [Google Scholar] [CrossRef]
- Amooaghaie, R.; Golmohammadi, S. Effect of Vermicompost on Growth, Essential Oil, and Health of Thymus vulgaris. Compost Sci. Util. 2017, 25, 166–177. [Google Scholar] [CrossRef]
- Yin, W.; Chai, Q.; Zhao, C.; Yu, A.; Fan, Z.; Hu, F.; Fan, H.; Guo, Y.; Coulter, J.A. Water Utilization in Intercropping: A Review. Agric. Water Manag. 2020, 241, 106335. [Google Scholar] [CrossRef]
- Chamkhi, I.; Cheto, S.; Geistlinger, J.; Zeroual, Y.; Kouisni, L.; Bargaz, A.; Ghoulam, C. Legume-Based Intercropping Systems Promote Beneficial Rhizobacterial Community and Crop Yield under Stressing Conditions. Ind. Crops Prod. 2022, 183, 114958. [Google Scholar] [CrossRef]
- Miyazawa, K.; Takeda, M.; Murakami, T.; Murayama, T. Dual and Triple Intercropping: Potential Benefits for Annual Green Manure Production. Plant Prod. Sci. 2014, 17, 194–201. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Javanmard, A.; Morshedloo, M.R.; Aghaee, A.; Maggi, F. Funneliformis mosseae Inoculation under Water Deficit Stress Improves the Yield and Phytochemical Characteristics of Thyme in Intercropping with Soybean. Sci. Rep. 2021, 11, 15279. [Google Scholar] [CrossRef]
- Duchene, O.; Vian, J.F.; Celette, F. Intercropping with Legume for Agroecological Cropping Systems: Complementarity and Facilitation Processes and the Importance of Soil Microorganisms. A Review. Agric. Ecosyst. Environ. 2017, 240, 148–161. [Google Scholar] [CrossRef]
- Grümberg, B.C.; Urcelay, C.; Shroeder, M.A.; Vargas-Gil, S.; Luna, C.M. The Role of Inoculum Identity in Drought Stress Mitigation by Arbuscular Mycorrhizal Fungi in Soybean. Biol. Fertil. Soils 2014, 51, 1–10. [Google Scholar] [CrossRef]
- de Andrade, S.A.L.; Domingues, A.P.; Mazzafera, P. Photosynthesis Is Induced in Rice Plants That Associate with Arbuscular Mycorrhizal Fungi and Are Grown under Arsenate and Arsenite Stress. Chemosphere 2015, 134, 141–149. [Google Scholar] [CrossRef]
- Bilalis, D.; Karkanis, A.; Efthimiadou, A.; Konstantas, A.; Triantafyllidis, V. Effects of Irrigation System and Green Manure on Yield and Nicotine Content of Virginia (Flue-Cured) Organic Tobacco (Nicotiana tabaccum), under Mediterranean Conditions. Ind. Crops Prod. 2009, 29, 388–394. [Google Scholar] [CrossRef]
- Mutsamba, E.F.; Nyagumbo, I.; Mupangwa, W. Forage and Maize Yields in Mixed Crop-Livestock Farming Systems: Enhancing Forage and Maize Yields in Mixed Crop-Livestock Systems under Conservation Agriculture in Sub-Humid Zimbabwe. NJAS-Wageningen J. Life Sci. 2020, 92, 100317. [Google Scholar] [CrossRef]
- Zhou, G.; Gao, S.; Lu, Y.; Liao, Y.; Nie, J.; Cao, W. Co-Incorporation of Green Manure and Rice Straw Improves Rice Production, Soil Chemical, Biochemical and Microbiological Properties in a Typical Paddy Field in Southern China. Soil Tillage Res. 2020, 197, 104499. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Ribeiro, N.P.; Assunção, N.S.; Geibel da Silva Nunes, J.; Sorroche, C.P.; Leonel, M. Impact of Nitrogen and Green Manure on Yield and Quality of Sweet Potato in Sandy Soil: A Brazilian Case Study. J. Agric. Food Res. 2021, 4, 100131. [Google Scholar] [CrossRef]
- Gao, S.; Cao, W.; Zhou, G.; Rees, R.M. Bacterial Communities in Paddy Soils Changed by Milk Vetch as Green Manure: A Study Conducted across Six Provinces in South China. Pedosphere 2021, 31, 521–530. [Google Scholar] [CrossRef]
- Bidgoli, R.D.; Mahdavi, M.J. Effect of Nitrogen and Two Types of Green Manure on the Changes in Percentage and Yield of Peppermint (Mentha piperita) Essential Oil. Not. Sci. Biol. 2018, 10, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Singh, A.; Singh, S.; Tripathi, R.S.; Singh, A.K.; Patra, D.D. Cowpea (Vigna unguiculata L. Walp.) as a Green Manure to Improve the Productivity of a Menthol Mint (Mentha arvensis L.) Intercropping System. Ind. Crops Prod. 2010, 31, 289–293. [Google Scholar] [CrossRef]
- Kaur, S.; Suseela, V. Unraveling Arbuscular Mycorrhiza-Induced Changes in Plant Primary and Secondary Metabolome. Metabolites 2020, 10, 335. [Google Scholar] [CrossRef]
- Merlin, E.; Melato, E.; Lourenço, E.L.B.; Jacomassi, E.; Junior, A.G.; da Cruz, R.M.S.; Otênio, J.K.; da Silva, C.; Alberton, O. Inoculation of Arbuscular Mycorrhizal Fungi and Phosphorus Addition Increase Coarse Mint (Plectranthus amboinicus Lour.) Plant Growth and Essential Oil Content. Rhizosphere 2020, 15, 100217. [Google Scholar] [CrossRef]
- Dos Santos Marques, C.T.; Gama, E.V.S.; da Silva, F.; Teles, S.; Caiafa, A.N.; Lucchese, A.M. Improvement of Biomass and Essential Oil Production of Lippia Alba (Mill) N.E. Brown with Green Manures in Succession. Ind. Crops Prod. 2018, 112, 113–118. [Google Scholar] [CrossRef]
- Jie, W.G.; Yao, Y.X.; Guo, N.; Zhang, Y.Z.; Qiao, W. Effects of Rhizophagus Intraradices on Plant Growth and the Composition of Microbial Communities in the Roots of Continuous Cropping Soybean at Maturity. Sustainability 2021, 13, 6623. [Google Scholar] [CrossRef]
- Klironomos, J.N.; Mouroglis, P.; Kendrick, B.; Widden, P. A Comparison of spatial heterogenecity of VAM fungi in two maple-forest soil. Can. J. Bot. 1993, 71, 1472–1480. [Google Scholar] [CrossRef]
- Jones, J.B. Micronutrients in Agriculture; Soil Science Society America: Madison, WI, USA, 1972. [Google Scholar]
- Tandon, H.L.S.; Cescas, M.P.; Tyner, E.H. An Acid-Free Vanadate-Molybdate Reagent for the Determination of Total Phosphorus in Soils. Soil Sci. Soc. Am. J. 1968, 32, 48–51. [Google Scholar] [CrossRef]




| Treatments | Plant Height (cm) | Number of Nodes per Plant | Number of Leaves per Plant | Number of Lateral Branches per Plant | Leaf Greenness (SPAD Index) | Dry Yield (g m−2) |
|---|---|---|---|---|---|---|
| Control | 45.1 e | 9.8 d | 478.7 d | 17.1 e | 34.9 c | 194.4 d |
| Bm | 47.3 e | 9.8 d | 511.3 d | 18.1 de | 35.9 c | 203.3 cd |
| HVm | 50.4 d | 11.7 c | 545 d | 19.8 cde | 36.3 bc | 217.7 c |
| 75%HV + 25%B | 50.6 d | 12.2 c | 625.8 c | 21.6 abc | 43 a | 240.8 b |
| 50%HV + 50%B | 61.5 a | 17.5 a | 706.3 ab | 24.4 a | 45.5 a | 266.7 a |
| 25%HV + 75%B | 54.1 c | 12.1 c | 685.8 abc | 22.6 abc | 40.5 abc | 249.3 ab |
| AMF | 58.6 b | 16.9 a | 737 a | 23.3 ab | 44.4 a | 259.5 ab |
| VC | 55.5 c | 15 b | 658.8 bc | 20.4 bcd | 42.4 ab | 251.7 ab |
| LSD | 2.51 | 1.86 | 69.58 | 3.15 | 6.31 | 19.84 |
| Treatments | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Components | RI | RI lit | C | Bm | HVm | 25%HV + 75%B | 50%HV + 50%B | 75%HV + 25%B | AMF | VC |
| 1 | α-Pinene | 931 | 932 | 0.3 | 0.36 | 0.32 | 0.54 | 0.30 | 0.51 | 0.47 | 0.36 |
| 2 | Sabinene | 970 | 969 | 0.58 | 0.31 | 0.49 | 0.43 | 0.18 | 0.36 | 0.44 | 0.39 |
| 3 | β-Pinene | 975 | 974 | 0.79 | 0.55 | 0.8 | 0.71 | 0.79 | 0.72 | 0.84 | 0.92 |
| 4 | Myrcene | 988 | 988 | 0.46 | 0.39 | 0.47 | 0.32 | 0.46 | 0.41 | 0.40 | 0.72 |
| 5 | 3-octanol | 1000 | 998 | 0.2 | 0.18 | 0.14 | 0.44 | 0.11 | 0.56 | 0.16 | 0.96 |
| 6 | α-Terpinene | 1017 | 1014 | 0.51 | 0.68 | 0.78 | 0.54 | 0.11 | 0.57 | 0.62 | 0.14 |
| 7 | Limonene | 1026 | 1024 | 2.91 | 0.84 | 1.16 | 2.92 | 1.91 | 1.31 | 1.82 | 2.52 |
| 8 | 1,8-cineole | 1029 | 1026 | 6.18 | 6.24 | 6.69 | 6.31 | 7.78 | 6.47 | 6.91 | 6.33 |
| 9 | γ-Terpinene | 1058 | 1054 | 0.58 | 0.08 | 0.19 | 0.78 | 0.58 | 0.52 | 0.22 | 0.21 |
| 10 | cis-Sabinene hydrate | 1066 | 1065 | 1.37 | 0.93 | 1.00 | 1.21 | 1.09 | 0.96 | 1.23 | 1.2 |
| 11 | Linalool | 1103 | 1095 | 0.74 | 0.42 | 0.43 | 0.30 | 0.44 | 0.43 | 0.42 | 0.43 |
| 12 | Menthone | 1152 | 1148 | 16.96 | 18.61 | 19.53 | 17.32 | 19.26 | 17.42 | 20.64 | 19.05 |
| 13 | Menthofuran | 1161 | 1159 | 3.14 | 3.82 | 2.28 | 3.10 | 1.14 | 2.97 | 2.29 | 1.83 |
| 14 | δ-Terpineol | 1162 | 1162 | 3.49 | 3.46 | 3.65 | 3.76 | 3.94 | 3.13 | 3.79 | 3.64 |
| 15 | neo-Menthol | 1163 | 1161 | 3.18 | 3.25 | 3.71 | 3.39 | 3.81 | 3.88 | 3.52 | 3.22 |
| 16 | Menthol | 1175 | 1167 | 32.35 | 34.91 | 35.77 | 35.11 | 37.73 | 36.91 | 36.11 | 35.63 |
| 17 | Terpinen-4-ol | 1177 | 1177 | 0.06 | 0.26 | 0.14 | 0.70 | 0.22 | 0.88 | 0.19 | 0.13 |
| 18 | neo-iso-Menthol | 1184 | 1184 | 3.41 | 3.68 | 4.00 | 3.21 | 3.81 | 3.77 | 3.83 | 3.59 |
| 19 | Pulegone | 1236 | 1233 | 3.05 | 2.35 | 2.13 | 2.18 | 2.05 | 2.01 | 2.09 | 2.11 |
| 20 | Piperitone | 1252 | 1252 | 2.54 | 1.96 | 2.2 | 2.51 | 1.54 | 2.46 | 1.36 | 1.49 |
| 21 | neo-Menthyl acetate | 1273 | 1271 | 0.88 | 0.76 | 0.6 | 0.95 | 0.47 | 0.57 | 0.45 | 0.57 |
| 22 | p-Menth-1-en-9-ol | 1294 | 1294 | 2.36 | 1.97 | 1.41 | 1.42 | 1.16 | 1.89 | 2.17 | 1.65 |
| 23 | iso-Menthyl acetate | 1307 | 1304 | 0.47 | 0.37 | 0.3 | 0.15 | 0.24 | 0.23 | 0.19 | 0.3 |
| 24 | β-Bourbonene | 1382 | 1387 | 0.87 | 0.43 | 0.22 | 0.31 | 0.17 | 0.41 | 0.1 | 0.62 |
| 25 | (E)-Caryophyllene | 1416 | 1417 | 2.29 | 2.59 | 2.11 | 1.96 | 2.19 | 2.35 | 2.66 | 2.29 |
| 26 | (E)-β-Farnesene | 1457 | 1454 | 0.68 | 0.13 | 0.23 | 0.34 | 0.45 | 0.50 | 0.93 | 0.49 |
| 27 | Germacrene D | 1479 | 1484 | 3.06 | 3.44 | 3.13 | 3.28 | 3.24 | 3.36 | 3.35 | 3.16 |
| 28 | Elixene | 1494 | 1492 | 1.44 | 1.31 | 1.05 | 0.88 | 0.9 | 1.08 | 0.41 | 1.12 |
| 29 | Viridiflorol | 1589 | 1592 | 0.77 | 0.25 | 0.63 | 0.15 | 0.86 | 0.7 | 0.18 | 0.33 |
| Total identified (%) | 95.62 | 94.53 | 95.56 | 95.22 | 96.93 | 97.34 | 97.79 | 95.4 |
| Texture | N (%) | P (mg kg−1) | K (mg kg−1) | pH | EC (dS m−1) | Organic Matter (%) |
|---|---|---|---|---|---|---|
| (Sandy clay loam) | 0.085 | 9.32 | 510 | 8.2 | 0.255 | 0.78 |
| Year | April | May | June | July | August | September | October |
|---|---|---|---|---|---|---|---|
| Monthly average temperature (°C) | |||||||
| 2018 | 12.6 | 16.6 | 24.1 | 30.2 | 27.7 | 23.6 | 15.9 |
| 2019 | 10.4 | 18.5 | 25.7 | 27.6 | 27.8 | 22.1 | 16.7 |
| 2020 | 11.8 | 19.1 | 24.2 | 28 | 25.1 | 23.8 | 16.1 |
| Total monthly precipitation (mm) | |||||||
| 2018 | 44.9 | 54.5 | 1.7 | 0.1 | 0 | 0 | 14.5 |
| 2019 | 51.3 | 37.8 | 4.2 | 0 | 0 | 0 | 6.3 |
| 2020 | 63.3 | 12 | 2.6 | 0.1 | 1.2 | 0 | 0.4 |
| Total Nitrogen (%) | P (%) | K (%) | pH | EC (µmhos.cm−1) | Organic Carbon (%) | Cu (ppm) | Zn (ppm) | Ca (ppm) |
|---|---|---|---|---|---|---|---|---|
| 1.6 | 0.47 | 0.27 | 7.84 | 1.64 | 7.13 | 3.84 | 66.6 | 8.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javanmard, A.; Amani Machiani, M.; Haghaninia, M.; Pistelli, L.; Najar, B. Effects of Green Manures (in the Form of Monoculture and Intercropping), Biofertilizer and Organic Manure on the Productivity and Phytochemical Properties of Peppermint (Mentha piperita L.). Plants 2022, 11, 2941. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11212941
Javanmard A, Amani Machiani M, Haghaninia M, Pistelli L, Najar B. Effects of Green Manures (in the Form of Monoculture and Intercropping), Biofertilizer and Organic Manure on the Productivity and Phytochemical Properties of Peppermint (Mentha piperita L.). Plants. 2022; 11(21):2941. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11212941
Chicago/Turabian StyleJavanmard, Abdollah, Mostafa Amani Machiani, Mohammad Haghaninia, Luisa Pistelli, and Basma Najar. 2022. "Effects of Green Manures (in the Form of Monoculture and Intercropping), Biofertilizer and Organic Manure on the Productivity and Phytochemical Properties of Peppermint (Mentha piperita L.)" Plants 11, no. 21: 2941. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11212941