Allelopathy and Allelochemicals of Solidago canadensis L. and S. altissima L. for Their Naturalization
Abstract
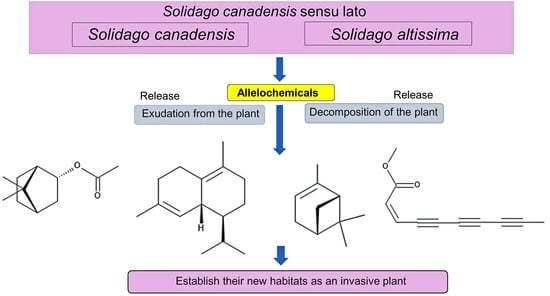
:1. Introduction
2. Allelopathy of S. canadensis
2.1. Allelopathy of Root Exudate and Plant Residue
2.2. Allelopathy of Plant Extract
2.3. Effects of the Extract on Arbuscular Mycorrhizal Fungi
| Source | Inhibition | Target Plant Species | Reference | |||
|---|---|---|---|---|---|---|
| Germination | Growth | Chlorophyll | Mycorrhizal colonization | |||
| Root exudate | ✓ | Gnaphalium affine, Xanthium sibiricum, Conyza canadensis, Celosia argentea, Aster subulatus, Sesbania cannabina, Eclipta prostrata | [52] | |||
| ✓ | Arabidopsis thaliana, | [53] | ||||
| Rhizosphere soil | ✓ | ✓ | Dactylis glomerata, Lythrum salicaria, Stachys officinalis, Trifolium pratense | [53] | ||
| Soil extract | ✓ | ✓ | Digitaria sanguinalis, Amaranthus retroflexus | [55] | ||
| Residue | ✓ | ✓ | Raphanus sativus, Triticum aestivum | [56] | ||
| Plant extract | ||||||
| Whole part | ✓ | ✓ | Kummerowia striata | [65] | ||
| Leaf | ✓ | ✓ | Raphanus sativus, Lactuca sativa | [57] | ||
| ✓ | ✓ | Triticum aestivum, Setaria viridi | [58] | |||
| ✓ | Raphanus sativus | [59] | ||||
| ✓ | Trifolium pratense | [60] | ||||
| Leaf and stem | ✓ | ✓ | Raphanus sativus, Triticum aestivum | [56] | ||
| Above-ground part | ✓ | ✓ | Lactuca sativa | [61] | ||
| ✓ | ✓ | Digitaria sanguinalis (L.) Scop. and Amaranthus retroflexus | [55] | |||
| Above-ground part, root | ✓ | ✓ | Zoysia japonica | [62] | ||
| Stem, root, blossom, seed | ✓ | ✓ | Brassica napus, Lolium perenne | [63] | ||
| Leaf, stem, rhizome | ✓ | ✓ | Morus alba, Pharbitis nil, Triticum aestivum, Brassica campestris | [66] | ||
| Root, rhizome | ✓ | Raphanus sativus, Lactuca sativa | [57] | |||
| ✓ | ✓ | Trifolium repens, Trifolium pratense, Medicago lupulina, Suaeda glauca, Plantago virginica, Kummerowia stipulacea, Festuca arundinacea, Ageratum conyzoides, Portulaca oleracea, Amaranthus spinosus | [69] | |||
| Rhizome | ✓ | Echinochloa crus-galli, Kummerowia striata, Ageratum conyzoides | [67] |
2.4. Allelochemicals
2.5. Contribution of Allelopathy of S. canadensis to Its Invasiveness
3. Allelopathy of S. altissima
3.1. Terpene
| Chemical Class | Compound | Solidado canadensis | Reference | Solidado altissima | Reference | |||
|---|---|---|---|---|---|---|---|---|
| Extract | Essential oil | Extract | Soil | Essential oil | ||||
| Fatty acid | 1: n-Hexadecanonic acid | ✓ | [56] | |||||
| Polyacetylene | 2: cis-Dehydromatricaria ester | ✓ | ✓ | [45,96,98,104] | ||||
| 3: trans-Dehydromatricaria ester | ✓ | [45] | ||||||
| 4: (2Z,8Z)-10-Tigloyloxy matricaria ester | ✓ | [95] | ||||||
| 5: (2Z,8Z)-10-Angeloyloxy matricaria ester | ✓ | [95,96] | ||||||
| 6: Dehydromatricaria lactone | ✓ | [95,104,111] | ||||||
| 7: (4Z,8Z)-10-Trigloyloxy matricaria lactone | ✓ | [95,111] | ||||||
| 8: (4Z,8Z)-10-Angeloyloxy matricaria lactone | ✓ | [95] | ||||||
| Monoterpene | 9: α-Pinene | ✓ | [83,84,85,86] | ✓ | ✓ | [33,98] | ||
| 10: β-Pinene | ✓ | [84] | ✓ | ✓ | [33,98] | |||
| 11: trans-Verbenol | ✓ | [83] | ||||||
| 12: Limonene | ✓ | [83,84,85,86] | ✓ | ✓ | [33,98] | |||
| 13: Sabinene | ✓ | [33] | ||||||
| 14: Myrcene | ✓ | [33] | ||||||
| 15: Thymol | ✓ | [84] | ||||||
| 16: Bornyl acetate | ✓ | [83] | ✓ | [33] | ||||
| Sesquiterpene | 16: β-Elemene | ✓ | [84] | |||||
| 17: (+)epi-Bicyclosesquiphellandrene | ✓ | [84] | ||||||
| 18: β-Cadinene | ✓ | [84] | ||||||
| 19: γ-Cadinene | ✓ | [84] | ||||||
| 20: δ-Cadinene | ✓ | [84] | ||||||
| 21: α-muurolene | ✓ | [84] | ||||||
| 22: γ-muurolene | ✓ | [84] | ||||||
| 23: Germacrene D | ✓ | [84,86] | ✓ | ✓ | [33,98] | |||
| 24: α-cubebene | ✓ | [84] | ||||||
| 25: β-Cubebene | ✓ | [83,84] | ||||||
| Diterpene | 26: 13E-kolavenic acid | ✓ | [95] | |||||
| 27: 13E-7α-acetoxyl kolavenic acid | ✓ | [95,108] | ||||||
| Polyphenol | 28: Chlorogenic acid | ✓ | [76] | |||||
| Flavonoid | 29: Kaempferol-3-O-D-glucoside | ✓ | [75] | |||||
| 30: Quercitrin | ✓ | [76,77] | ||||||
| 31: Rutin | ✓ | [76] | ||||||
3.2. Polyacetylene
3.3. Contribution of Allelopathy of S. altissima to Its Invasiveness
4. Conclusions
Funding
Institutional Review Statement
Informed Consent Statement
Conflicts of Interest
References
- Weber, E. The dynamics of plant invasions: A case study of three exotic goldenrod species (Solidago L.) in Europe. J. Biogeogr. 1998, 25, 147–154. [Google Scholar] [CrossRef]
- Weber, E. Morphological variation of the introduced perennial Solidago canadensis L. sensu lato (Asteraceae) in Europe. Bot. J. Linn. Soc. 1997, 123, 197–210. [Google Scholar] [CrossRef]
- Weber, E. Biological flora of Central Europe: Solidago altissima L. Flora 2000, 195, 123–134. [Google Scholar] [CrossRef]
- Invasive Species Compendium, Solidago canadensis. Available online: https://www.cabi.org/isc/datasheet/50599 (accessed on 12 September 2022).
- Royal Botanical Gardens, Kew, Solidago canadensis. Solidago altissima L. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:249454-1 (accessed on 12 September 2022).
- Royal Botanical Gardens, Kew, Solidago altissima L. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:285347-2 (accessed on 12 September 2022).
- Croat, T. Solidago canadensis complex of the great plains. Brittonia 1972, 24, 317–326. [Google Scholar] [CrossRef]
- Werner, P.A.; Bradbury, I.K.; Gross, R.S. The biology of Canadian weeds. 45. Solidago canadensis L. Can. J. Plant Sci. 1980, 60, 1393–1409. [Google Scholar] [CrossRef]
- Curtis, J.D.; Lersten, N.R. Oil reservorirs in stem, rhizomes, and root of Solidago canadensis (Asteraceas, tribe Astereae). Nor. J. Bot. 1990, 10, 443–449. [Google Scholar] [CrossRef]
- Meyer, A.H.; Schmid, B. Seed dynamics and seedling establishment in the invading perennial Solidago altissima under different experimental treatments. J. Ecol. 1999, 87, 28–41. [Google Scholar] [CrossRef]
- Cornelius, R. The strategies of Solidago canadensis L. in relation to urban habitats. I. Resource requirements. Acta Oecol. 1990, 11, 19–34. [Google Scholar]
- Huang, H.; Guo, S.; Chen, G. Reproductive biology in an invasive plant Solidago canadensis. Front. Biol. China 2007, 2, 196–204. [Google Scholar] [CrossRef]
- Follak, S.; Eberius, M.; Essl, F.; Fürdös, A.; Sedlacek, N.; Trognitz, F. Invasive alien plants along roadsides in Europe. EPPO Bull. 2018, 48, 256–265. [Google Scholar] [CrossRef]
- Park, J.S.; Choi, D.; Kim, Y. Potential Distribution of Goldenrod (Solidago altissima L.) during Climate Change in South Korea. Sustainability 2020, 12, 6710. [Google Scholar] [CrossRef]
- Kotowska, D.; Pärt, T.; Żmihorski, M. Evaluating google street view for tracking invasive alien plants along roads. Ecol. Indi. 2021, 121, 107020. [Google Scholar] [CrossRef]
- Shimoda, M.; Nakamoto, M. Vegetation and threatened plant dynamics of wet abandoned rice fields in Nakaikemi, Fukui, Prefecture, Japan. Jpn. J. Ecol. 2003, 53, 197–217. [Google Scholar]
- Shimoda, S.; Wagai, R. Ecosystem dynamics after abandonment of rice paddy fields; Does aaien plant invasion enhance carbon storage? Ecosystems 2020, 23, 617–629. [Google Scholar] [CrossRef]
- Newell, S.J.; Tramer, E.J. Reproductive strategies in herbaceous plant communities during succession. Ecology 1978, 59, 228–234. [Google Scholar] [CrossRef]
- Bakelaar, R.G.; Odum, E.P. Community and population level response to fertilization in an old-field ecosystem. Ecology 1978, 59, 660–665. [Google Scholar] [CrossRef]
- Maddox, G.D.; Cook, R.E.; Wimberger, P.H.; Gardescu, S. Clone structure in four Solidago altissima (Asteraceae) population: Rhizome connections within genotypes. Am. J. Bot. 1989, 76, 318–326. [Google Scholar] [CrossRef]
- Dong, L.J.; Yu, H.W.; He, W.M. What determines positive, neutral and negative impacts of Solidago canadensis invasion on native plant species richness? Sci. Rep. 2015, 5, 16804. [Google Scholar] [CrossRef] [Green Version]
- Fenesi, A.; Vágási, C.I.; Beldean, M.; Földesi, R.; Kolcsár, L.P.; Shapiro, J.T.; Török, E.; Kovács-Hostyánszki, A. Solidago canadensis impacts on native plant and pollinator communities in different-aged old fields. Basic Appl. Ecol. 2015, 16, 335–346. [Google Scholar] [CrossRef] [Green Version]
- Zagurskaya, Y.V. Study issues of invasive species of the genus Solidago. Ecosyst. Transform. 2022, 5, 42–54. [Google Scholar] [CrossRef]
- EPPO. PQR database. Paris, France: European and Mediterranean Plant Protection Organization. Available online: https://gd.eppo.int/taxon/SOOCA (accessed on 12 September 2022).
- Weber, E.; Schmid, B. Latitudinal population differentiation in two species of Solidago (Asteraceae) introduced into Europe. Am. J. Bot. 1998, 85, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- iNatuarist, Identifying Solidago altissima & Solidago canadensis. Available online: https://www.inaturalist.org/posts/19288-identifying-solidago-altissima-solidago canadensis (accessed on 12 September 2022).
- Szymura, M.; Szymura, T.H. Interactions between alien goldenrods (Solidago and Euthamia species) and comparison with native species in Central Europe. Flora 2016, 218, 51–61. [Google Scholar] [CrossRef]
- Semple, J.C.; Rahman, H.; Bzovsky, S.; Sorour, M.K.; Kornobis, K.; Laphitz, R.L.; Tong, L. A multivariate morphometric study of the Solidago altissima complex and S. canadensis (Asteraceae: Astereae). Phytoneuron 2015, 10, 1–31. [Google Scholar]
- Verloove, F.; Zonneveld, B.J.M.; Semple, J.C.S. First evidence for the presence of invasive Solidago altissima (Asteraceae) in Europe. Willdenowia 2017, 47, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Melville, M.R.; Morton, J.K. A biosystematics study of the Solidago canadensis (Compositae) complex. I. The Ontario populations. Can. J. Bot. 1982, 60, 976–997. [Google Scholar] [CrossRef]
- Szymura, M.; Szymura, T.H.; Kreitschitz, A. Morphological and cytological diversity of goldenrods (Solidago L. and Euthamia Nutt.) from south-western Poland. Biodiv. Res. Conserv. 2015, 38, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Pisula, N.; Meiners, S.J. Allelopatyhic effcts of goldenrod species on turnover in successional communities. Am. Midl. Nat. 2010, 163, 161–172. [Google Scholar] [CrossRef]
- Lawson, S.K.; Sharp, L.G.; Powers, C.N.; McFeeters, R.L.; Satyal, P.; Setzer, W.N. Volatile Compositions and Antifungal Activities of Native American Medicinal Plants: Focus on the Asteraceae. Plants 2020, 9, 126. [Google Scholar] [CrossRef] [Green Version]
- Callaway, R.M.; Aschehoug, E.T. Invasive plants versus their new and old neighbors: A mechanism for exotic invasion. Science 2000, 290, 521–523. [Google Scholar] [CrossRef]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 419–426. [Google Scholar] [CrossRef]
- Cappuccino, N.; Arnason, J.T. Novel chemistry of invasive exotic plants. Biol. Lett. 2006, 2, 189–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chengxu, W.; Mingxing, Z.; Xuhui, C.; Bo, Q. Review on allelopathy of exotic invasive plants. Procedia. Engin. 2011, 18, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Meiners, S.J.; Kong, C.H.; Ladwig, L.M.; Pisula, N.L.; Lang, K.A. Developing an ecological context for allelopathy. Plant. Ecol. 2012, 213, 1861–1867. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Involvement of allelopathy in the invasive potential of Tithonia diversifolia. Plants 2020, 9, 766. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy of Lantana camara as an invasive plant. Plants 2021, 10, 1028. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Allelopathy of knotweeds as invasive plants. Plants 2022, 11, 3. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy and allelochemicals of Leucaena leucocephala as an invasive plant species. Plants 2022, 11, 1672. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Allelopathy and Allelochemicals of Imperata cylindrica as an invasive plant species. Plants 2022, 11, 2551. [Google Scholar] [CrossRef]
- Fisher, R.F.; Woods, R.A.; Glavicic, M.R. Allelopathic effects of golderrod and aster on young sugar maple. Can. J. Forest Res. 1978, 8, 1–9. [Google Scholar] [CrossRef]
- Kobayashi, A.; Morimoto, S.; Shibata, Y.; Yamashita, K.; Numata, M. C10-Polyacetylenes as allelopathic substances in dominants in early stages of secondary succession. J. Chem. Ecol. 1980, 6, 119–131. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; pp. 1–422. [Google Scholar]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonanomi, G.; Sicurezza, M.G.; Caporaso, S.; Esposito, A.; Mazzoleni, S. Phytotoxicity dynamics of decaying plant materials. New Phytol. 2006, 169, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Belz, R.G. Allelopathy in crop/weed interactions—An update. Pest. Manag. Sci. 2007, 63, 308–326. [Google Scholar] [CrossRef] [PubMed]
- Bielecka, A.; Borkowska, L.; Królak, E. Environmental changes caused by the clonal invasive plant Solidago canadensis. Ann. Bot. Fennici. 2019, 57, 33–48. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, H.; Wanga, S.; Weia, M.; Du, D. Plant community and the influence of plant taxonomic diversity on community stability and invasibility: A case study based on Solidago canadensis L. Sci. Total Environ. 2021, 768, 144518. [Google Scholar] [CrossRef]
- Yang, B.; Li, J. Phytotoxicity of toot exudates of invasive Solidago canadensis on co-occurring native and invasive plant species. Pak. J. Bot. 2022, 54, 1019–1024. [Google Scholar] [CrossRef]
- Abhilasha, D.; Quintana, N.; Vivanco, J.; Joshi, J. Do allelopathic compounds in invasive Solidago canadensis s.l. restrain the native European flora? J. Ecol. 2008, 96, 993–1001. [Google Scholar] [CrossRef]
- Weißhuhn, K.; Prati, D. ; Activated carbon may have undesired side effects for testing allelopathy in invasive plants. Basic Appl. Ecol. 2009, 10, 500–507. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, J.; Yao, B.; Lu, H.; Zhang, Y.; Xu, J.; Song, X.; Qiang, S. Polyploidy-promoted phenolic metabolism confers the increased competitive ability of Solidago canadensis. Oikos 2021, 130, 1014–1025. [Google Scholar] [CrossRef]
- Zhang, D.; Ye, Y.; Li, L.; Domg, L. Allelopathic pathways, isolation and identification of an allelopathic substance from Solidago canadensis L. Allelopath. J. 2014, 33, 201–212. [Google Scholar]
- Butcko, V.M.; Jensen, R.J. Evidence of tissue-specific allelopathic activity in Euthamia graminifolia and Solidago canadensis (Asteraceae). Am. Midl. Nat. 2002, 148, 253–262. [Google Scholar] [CrossRef]
- Li, S.L.; Li, Z.H.; Wang, Y.F.; Xiao, R.; Pan, C.D.; Wang, Q. Preliminary study for the allelopathic effect of water extracts from Solidago canadensis leaves. Adv. Mater. Res. 2013, 699, 340–348. [Google Scholar] [CrossRef]
- Możdżeń, K.; Barabasz-Krasny, B.; Zandi, P.; Kliszcz, A.; Puła, J. Effect of aqueous extracts from Solidago canadensis L. Leaves on germination and early growth stages of three cultivars of Raphanus Sativus L. var. Radicula Pers. Plants 2020, 9, 1549. [Google Scholar] [CrossRef] [PubMed]
- Zandi, P.; Barabasz-Krasny, B.; Stachurska-Swakoń, A.; Puła, J.; Możdżeń, K. Allelopathic effect of invasive Canadian goldenrod (Solidago canadensis L.) on early growth of red clover (Trifolium pratense L.). Not. Bot. Horti. Agrobo. 2020, 48, 2060–2071. [Google Scholar] [CrossRef]
- Yu, Y.; Cheng, H.; Xu, Z.; Zhong, S.; Wang, C.; Guo, E. Invasion intensity modulates the allelopathic impact of Solidago canadensis L. leaves and roots against Lactuca sativa L. during germination and early seedling stage. Int. J. Environ. Res. 2022, 16, 48. [Google Scholar] [CrossRef]
- Sun, J.F.; Liang, Q.J.; Wu, N.; Javed, Q.; Huang, P.; Du, D.L. Allelopathic effects of aqueous extracts from different plant parts of Canada goldenrod (Solidago canadensis L.) on seed germination and seedling growth of Korean lawngrass (Zoysia japonica Steud.). Appl. Ecol. Environ. Res. 2022, 20, 1009–1022. [Google Scholar] [CrossRef]
- Baležentien, L. Secondary metabolite accumulation and phytotoxicity of invasive species Solidago canadensis L. during the growth period. Allelopath. J. 2015, 35, 217–226. [Google Scholar]
- Yang, R.Y.; Mei, L.X.; Tang, J.J.; Chen, X. Allelopathic effects of invasive Solidago canadensis L. on germination and growth of native Chinese plant species. Allelopath. J. 2007, 19, 241–248. [Google Scholar]
- Yuan, Y.; Wang, B.; Zhang, S.; Tang, J.; Tu, C.; Hu, S.; Yong, J.W.H.; Chen, X. Enhanced allelopathy and competitive ability of invasive plant Solidago canadensis in its introduced range. J. Plant Ecol. 2013, 6, 253–263. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.Y.; Tan, J.Z.; Wan, Z.G.; Gu, F.G.; Zhu, M.D. Allelopathic effects of extracts from Solidago canadensis L. against seed germination and seedling growth of some plants. J. Environ. Sci. 2006, 18, 304–309. [Google Scholar]
- Zhang, Q.; Yao, L.J.; Yang, R.Y.; Yang, X.Y.; Tang, J.J.; Chin, X. Potential allelopathic effects of an invasive species Solidago canadensis on the mycorrhizae of native plant species. Allelopath. J. 2007, 20, 71–78. [Google Scholar]
- Sun, Z.K.; He, W.M. Evidence for enhanced mutualism hypothesis: Solidago canadensis plants from regular soils perform better. PLoS ONE 2010, 5, e15418. [Google Scholar] [CrossRef]
- Betekhtina, A.A.; Mukhacheva, T.A.; Kovaleva, S.Y.; Gusevb, A.P.; Veselkin, D.V. Abundance and diversity of arbuscular mycorrhizal fungi in invasive Solidago canadensis and indigenous S. virgaurea. Russ. J. Ecol. 2016, 47, 575–579. [Google Scholar] [CrossRef]
- Dong, L.J.; Ma, L.N.; He, W.M. Arbuscular mycorrhizal fungi help explain invasion success of Solidago canadensis. Appl. Soil. Ecol. 2021, 167, 103763. [Google Scholar] [CrossRef]
- Řezáčová, V.; Řezáč, M.; Gryndler, M.; Hršelová, H.; Gryndlerová, H.; Michalová, T. Plant invasion alters community structure and decreases diversity of arbuscular mycorrhizal fungal communities. Appl. Soil. Ecol. 2021, 167, 104039. [Google Scholar] [CrossRef]
- Yang, R.; Zhou, G.; Zan, S.; Guo, F.; Su, N.; Li, J. Arbuscular mycorrhizal fungi facilitate the invasion of Solidago canadensis L. in southeastern China. Acta Oecol. 2014, 61, 74–77. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, Y.; Tang, J.; Chen, X. The invasive plant Solidago canadensis L. Suppresses local soil pathogens through allelopathy. Appl. Soil. Ecol. 2009, 41, 215–222. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, W.; Wang, B.; Tang, J.; Chen, X. Secondary metabolites from the invasive Solidago canadensis L. Accumulation in soil and contribution to inhibition of soil pathogen Pythium ultimum. Appl. Soil. Ecol. 2011, 48, 280–286. [Google Scholar] [CrossRef]
- Jun, L.; Yonghao, Y.; Hongwu, H.; Liyao, D. Kaempferol-3-O-β-D-glucoside, a potential allelochemical isolated from Solidago canadensis. Allelopath. J. 2011, 28, 259–266. [Google Scholar]
- Radusiene, J.; Marska, M.; Ivanauskas, L.; Jakstas, J.; Karpaviciene, B. Assessment of phenolic compound accumulation in two widespread goldenrods. Ind. Crops Prod. 2015, 63, 158–166. [Google Scholar] [CrossRef]
- Likhanov, A.; Oliinyk, M.; Pashkevych, N.; Churilov, A.; Kozyr, M. The role of flavonoids in invasion strategy of Solidago canadensis L. Plants 2021, 10, 1748. [Google Scholar] [CrossRef]
- Shelepova, O.; Vinogradova, Y.; Vergun, O.; Grygorieva, O.; Brindza, J. Assessment of flavonoids and phenolic compound accumulation in invasive Solidago сanadensis L. in Slovakia. Slovak J. Food Sci. 2020, 14, 587–594. [Google Scholar]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Jassbi, A.R.; Zare, S.; Firuzi, O.; Xiao, J. Bioactive phytochemicals from shoots and roots of Salvia species. Phytochem Rev. 2016, 15, 829–867. [Google Scholar] [CrossRef]
- Macías, F.A.; Mejías, F.J.R.; Molinillo, J.M.G. Recent advances in allelopathy for weed control: From knowledge to applications. Pest Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef] [PubMed]
- Baranová, B.; Troščáková-Kerpčárová, E.; Grul’ová, D. Survey of the Solidago canadensis L. morphological traits and essential oil production: Aboveground biomass growth and abundance of the invasive goldenrod appears to be reciprocally enhanced within the invaded dtands. Plants 2022, 11, 535. [Google Scholar] [CrossRef]
- Radušienė, J.; Karpavičienė, B.; Marksa, M.; Ivanauskas, L.; Raudonė, L. Distribution patterns of essential oil terpenes in native and invasive Solidago species and their comparative assessment. Plants 2022, 11, 1159. [Google Scholar] [CrossRef]
- Grul’ová, D.; Baranová, B.; Ivanova, V.; De Martino, L.; Mancini, E.; De Feo, V. Composition and bio activity of essential oils of Solidago spp. and their Impact on radish and garden cress. Allelopath. J. 2016, 39, 129–141. [Google Scholar]
- Shelepova, O.; Vinogradova, Y.; Zaitchik, B.; Ruzhitsky, A.; Grygorieva, O.; Brindza, J. Constituents of the essential oil in Solidago canadensis L. from Eurasia. Slovak J. Food Sci. 2018, 12, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Marinas, I.C.; Oprea, E.; Buleandra, M.; Bleots, C.; Badea, I.R.; Anastasiu, P.; Lazar, V.; Gardus, I.D.; Chifiric, M.C. Chemical, antimicrobial, antioxidant and anti-proliferative features of the essential oil extracted from the invasive plant Solidago canadensis L. Rev. Chim. 2020, 71, 255–264. [Google Scholar] [CrossRef]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef]
- Chowhan, N.; Singh, H.P.; Batish, D.R.; Kohli, R.H. Phytotoxic effects of β-pinene on early growth and associated biochemical changes in rice. Acta Physiol. Plant. 2011, 33, 2369–2376. [Google Scholar] [CrossRef]
- Chowhan, N.; Singh, H.P.; Batish, D.R.; Kaur, S.; Ahuja, N.; Kohli, R.K. β-Pinene inhibited germination and early growth involves membrane peroxidation. Protoplasma 2013, 250, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.W.; Kil, B.S.; Han, D.M. Phytotoxic and antimicrobial activity of volatile constituents of Artemisia princeps var. orientalis. J. Chem. Ecol. 1993, 19, 2757–2766. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.P.; Vepachedu, R.; Gilroy, S.; Callaway, R.M.; Vivanco, J.M. Allelopathy and exotic plant invasion: From molecules and genes to species interaction. Science 2003, 301, 1377–1380. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008; pp. 1–815. [Google Scholar]
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoonoff, S. Roles of Arbuscular Mycorrhizal Fungi on Plant Growth and Performance: Importance in Biotic and Abiotic Stressed Regulation. Diversity 2020, 12, 370. [Google Scholar] [CrossRef]
- Tang, H.; Hassan, M.U.; Feng, L.; Nawaz, M.; Shah, A.N.; Qari, S.H.; Liu, Y.; Miao, J. The critical role of arbuscular mycorrhizal fungi to improve drought tolerance and nitrogen use efficiency in crops. Front. Plant Sci. 2022, 13, 919166. [Google Scholar] [CrossRef] [PubMed]
- Nishidono, Y.; Tanaka, K. Comprehensive characterization of polyacetylenes and diterpenes from the underground parts of Solidago altissima L. and their contribution to the overall allelopathic activity. Phytochemistry 2022, 193, 112986. [Google Scholar] [CrossRef]
- Ichihara, K.; Kawai, T.; Kaji, M.; Noda, M. A mew polyacetylene from Solidago altissima L. Agric. Biol. Chem. 1976, 40, 353–358. [Google Scholar] [CrossRef]
- Kawazu, K.; Ariwa, M.; Kii, Y. An ovicidal substance, cis-dehydromatricaria ester from Solidago altissima. Agric. Biol. Chem. 1977, 41, 223–224. [Google Scholar]
- Johnson, R.H.; Halitschke, R.; Kessler, A. Simultaneous analysis of tissue- and genotype-specific variation in Solidago altissima (Asteraceae) rhizome terpenoids, and the polyacetylene dehydromatricaria ester. Chemoecology 2010, 20, 255–264. [Google Scholar] [CrossRef]
- Nishino, C.; Manabe, S.; Kazui, M.; Matsuzaki, T. Piscicidal cis-clerodane diterpenes from Solidago altissima. L.: Absolute configurations of 5α, 10α-cis-clerodanes. Tetrahedron Lett. 1984, 25, 2809–2812. [Google Scholar] [CrossRef]
- Bohlmann, F.; Singh, P.; Singh, R.K.; Joshi, K.C.; Jakupovic, J. A diterpene with a new carbon skeleton from Solidago altissima. Phytochemistry 1985, 24, 1114–1115. [Google Scholar] [CrossRef]
- Tori, M.; Katto, A.; Sono, M. Nine new clerodane diterpenoids from rhizomes of Solidago altissima. Phytochemistry 1999, 52, 487–493. [Google Scholar] [CrossRef]
- Nishidono, Y.; Tanaka, K. New clerodane diterpenoids from Solidago altissima and stereochemical elucidation via 13C NMR chemical shift analysis. Tetrahedron 2022, 110, 132691. [Google Scholar] [CrossRef]
- Okano, A.; Nomura, Y.; Tezuka, T. Identification of bauerenol in Solidago altissima. J. Nat. Prod. 1983, 46, 750–751. [Google Scholar] [CrossRef]
- Sawabe, A.; Minemoto, K.; Minematsu, T.; Ouchi, S.; Okamoto, T.; Morita, M.; Ouchi, S.; Okamoto, T. Characterization of the Z and E isomers of dehydromatricaria lactones. J. Jpn. Oil Chem. Soc. 1997, 46, 413–417. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Tanaka, T.; Kouno, I.; Ishimaru, K. A new kaempferol trioside from Solidago altissima L. J. Nat. Med. 2007, 61, 351–354. [Google Scholar] [CrossRef]
- Wu, B.; Takahashi, T.; Kashiwagi, T.; Tebayashi, S.; Kim, C.S. New flavonoid glycosides from the leaves of Solidago altissima. Chem. Pharm. Bull. 2007, 55, 815–816. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Ogino, K.; Fujioka, T.; Yoshida, M.; Ishimaru, K. A new acylphloroglucinol glycoside from Solidago altissima L. J. Nat. Med. 2008, 62, 199–201. [Google Scholar] [CrossRef]
- Sawabe, A.; Minemoto, K.; Ouchi, S.; Okamoto, T. Effects of acetylenes and terpenoids from Solidago altissima L. on seed germination. J. Jpn. Oil Chem. Soc. 1999, 48, 139–142. [Google Scholar] [CrossRef] [Green Version]
- Salah, M.A.; Bedir, E.; Toyang, N.J.; Khan, I.A.; Harries, M.D.; Wedge, D.E. Antifungal clerodane diterpenes from Macaranga monandra (L) Muell. et Arg. (Euphorbiaceae). J. Agric. Food Chem. 2003, 51, 7607–7610. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M. Chemical defense against insects in Heterotheca subaxillaris and three Orobanchaceae species using exudates from trichomes. Pest Manag. Sci. 2019, 75, 2474–2481. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, K.; Kawai, T.; Noda, M. Polyacetylenes of Solidago altissima L. Agric. Biol. Chem. 1978, 42, 427–431. [Google Scholar] [CrossRef]
- Minto, R.E.; Blacklock, B.J. Biosynthesis and function of polyacetylenes and allied natural products. Prog. Lipid Res. 2008, 47, 233–306. [Google Scholar] [PubMed] [Green Version]
- Uesugi, A.; Kessler, K. Herbivore exclusion drives the evolution of plant competitiveness via increased allelopathy. New Phytol. 2013, 198, 916–924. [Google Scholar] [CrossRef]
- Carson, W.; Root, R. Herbivory and plant species coexistence: Community regulation by an outbreaking phytophagous insect. Ecol. Monogr. 2000, 70, 73–99. [Google Scholar] [CrossRef]
- Yamada, T. Miscanthus. In Industrial Crops. Handbook of Plant Breeding; Cruz, V.M.V., Dierig, D.A., Eds.; Springer: New York, NY, USA, 2015; Volume 9, pp. 43–66. [Google Scholar]
- Uesugi, A.; Johnson, R.; Kessler, K. Context-dependent induction of allelopathy in plants under competition. Oikos 2019, 128, 1492–1502. [Google Scholar] [CrossRef]
- Ito, I.; Kobayashi, K.; Yoneyama, T. Fate of dehydromatricaria ester added to soil and its implications for the allelopathic effect of Solidago altissima L. Ann. Bot. 1998, 82, 625–630. [Google Scholar] [CrossRef] [Green Version]
- Kaur, H.; Kaur, R.; Kaur, S.; Baldwin, I.T.; Inderjit. Taking ecological function seriously: Soil microbial communities can obviate allelopathic effects of released metabolites. PLoS ONE 2004, 4, e4700. [Google Scholar] [CrossRef]
- Kobayashi, K.; Koyama, H.; Shim, I. Relationship between behaviour of dehydromatricaria ester in soil and the allelopathic activity of Solidago altissima L. in the laboratory. Plant Soil 2004, 259, 97–102. [Google Scholar] [CrossRef]
- Tsao, R.; Eto, M. Light-activated plant growth inhibitory activity of cis-dehydromatricaria ester, rose bengal and fluoren-9-one on lettuce (Lactuca sativa L.). Chemosphere 1996, 32, 1307–1317. [Google Scholar] [CrossRef]
- Sakai, H.; Yoneda, K. Possible dual roles of an allelopathic compound, cis-dehydromatricaria ester. J. Chem. Ecol. 1981, 8, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Mack, R.M. Predicting the identity and fate of plant invaders: Emergent and emerging approaches. Biol. Conserv. 1996, 78, 107–121. [Google Scholar] [CrossRef]
- Keane, R.M.; Crawley, M.J. Exotic plant invsions and the enemy release hypothesis. Trends Ecol. Evol. 2002, 17, 164–169. [Google Scholar] [CrossRef]
- Ismail, M.; Kowsar, A.; Javed, S.; Choudhary, M.I.; Khan, S.W.; Abbas, Q.; Tang, Y.; Wang, W. The antibacterial, insecticidal and nematocidal activities and toxicity studies of Tanacetum falconeri Hook.f. Turk. J. Pharm. Sci. 2021, 18, 744–751. [Google Scholar]



| cis-DME Concentration (ppm) | Target Plant | Germination | Growth | Reference |
|---|---|---|---|---|
| (% of Control) | ||||
| 32 | Asclepias syriaca | 5 | [98] | |
| 48 | Poa pratensis | 20 | 50 | [113] |
| 50 | Oryza sativa | 88 | 10–15 | [45] |
| 50 | Ambrosia artemisiifolia | 25 | [45] | |
| 50 | Miscanthus sinensis | 22 | [45] | |
| 100 | Lactuca sativa | 30–56 | [108] | |
| 100 | Panicum crus-galli | 18 | [111] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato-Noguchi, H.; Kato, M. Allelopathy and Allelochemicals of Solidago canadensis L. and S. altissima L. for Their Naturalization. Plants 2022, 11, 3235. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11233235
Kato-Noguchi H, Kato M. Allelopathy and Allelochemicals of Solidago canadensis L. and S. altissima L. for Their Naturalization. Plants. 2022; 11(23):3235. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11233235
Chicago/Turabian StyleKato-Noguchi, Hisashi, and Midori Kato. 2022. "Allelopathy and Allelochemicals of Solidago canadensis L. and S. altissima L. for Their Naturalization" Plants 11, no. 23: 3235. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11233235