Multiple Autoregulation of Nodulation (AON) Signals Identified through Split Root Analysis of Medicago truncatula sunn and rdn1 Mutants
Abstract
:1. Introduction
2. Results and Discussion
2.1. AON Mutants Lack an Early Systemic Response to Previous Nodulation Events, but Suppression Returns after 10–15 Days

2.2. AON Mutants and Wild Type Fix Equivalent Amounts of Nitrogen at Time Points when Systemic Suppression Is Observed

2.3. Nitrate Reduces Nodulation in Both AON Mutants and Wild Type Plants in this System

2.4. Inoculation with Fix− or Nod+/Fix− Rhizobia Did Not Elicit the AON Response in all Plants

2.5. Nutrient Stress Causes Suppression of Nodulation in Wild Type and AON Mutants


3. Experimental Section
3.1. Plant Materials and Growth Conditions
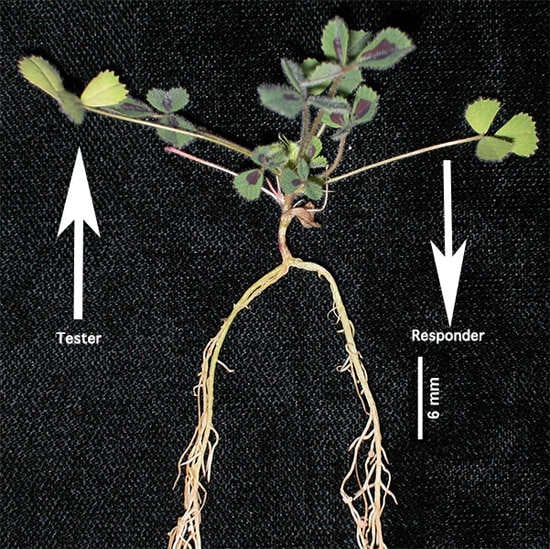
3.2. Split Root Development and Inoculation
3.3. Rhizobial Strains and Growth Conditions
3.4. Nitrogen Fixation Measurements
3.5. Determination of C and N
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Oldroyd, G.; Downie, A. Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu. Rev. Plant Biol. 2008, 59, 519–545. [Google Scholar] [CrossRef] [PubMed]
- Crawford, N.M.; Kahn, M.L.; Leustek, T.; Long, S.R. Nitrogen and sulfur. In Biochemistry and Molecular Biology of Plants; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; American Association of Plant Physiologists: Rockville, MD, USA, 2000; pp. 787–849. [Google Scholar]
- Kosslak, R.M.; Bohlool, B.B. Suppression of nodule development of one side of a split root system of soybeans caused by prior inoculation of the other side. Plant Physiol. 1984, 75, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, T.V.; Bhagwat, A.A.; Bauer, W. Transient susceptibility of root-cells in 4 common legumes to nodulation by rhizobia. Plant Physiol. 1981, 68, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Hara, H.; Kinoue, T.; Abe, M.; Uchiumi, T.; Kucho, K.; Higashi, S.; Hirsch, A.M.; Arima, S. Split-root study of autoregulation of nodulation in the model legume Lotus japonicus. J. Plant Res. 2008, 121, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Sargent, L.; Huang, S.Z.; Rolfe, B.G.; Djordjevic, M.A. Split-root assays using Trifolium subterraneum show that rhizobium infection induces a systemic response that can inhibit nodulation of another invasive rhizobium strain. Appl. Environ. Microbiol. 1987, 53, 1611–1619. [Google Scholar] [PubMed]
- Van Brussel, A.A.N.; Tak, T.; Boot, K.J.M.; Kijne, J.W. Autoregulation of root nodule formation: Signals of both symbiotic partners studied in a split-root system of Vicia sativa subsp. Nigra. Mol. Plant-Microbe Interact. 2002, 15, 341–349. [Google Scholar] [CrossRef]
- Krusell, L.; Madsen, L.H.; Sato, S.; Aubert, G.; Genua, A.; Szczyglowski, K.; Duc, G.; Kaneko, T.; Tabata, S.; De Bruijn, F.J.; et al. Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature 2002, 420, 422–426. [Google Scholar]
- Nishimura, R.; Hayashi, M.; Wu, G.J.; Kouchi, H.; Imaizumu-Anraku, H.; Murakami, Y.; Kawasaki, S.; Akao, S.; Ohmori, M.; Nagasawa, M.; Pajuelo, E.; Sandal, N.; Stougaard, J. HAR1 mediates systemic regulation of symbiotic organ development. Nature 2002, 420, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Searle, I.R.; Men, A.E.; Laniya, T.; Buzas, D.; Iturbe-Ormaetxe, I.; Carroll, B.J.; Gresshoff, P.M. Long-distance signaling in nodulation directed by a clavata1-like receptor kinase. Science 2003, 299, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, E.; Journet, E.P.; Carvalho-Niebel, F.; Duc, G.; Frugoli, J. The Medicago truncatula SUNN gene encoding a CLV1-like leucine-rich repeat receptor kinase regulates both nodule number and root length. Plant Mol. Biol. 2005, 58, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Shinohara, H.; Mori, T.; Matsubayashi, Y.; Kawaguchi, M. Root-derived cle glycopeptides control nodulation by direct binding to HAR1 receptor kinase. Nat. Commun. 2013, 4, 2191. [Google Scholar]
- Ferguson, B.J.; Indrasmunar, A.; Hayashi, S.; Lin, M.H.; Reid, D.E.; Gresshoff, P.M. Molecular analysis of legume nodule development and autoregulation. J. Integr. Plant Biol. 2010, 52, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, E.; Kassaw, T.; Smith, L.; Marsh, J.; Oldroyd, G.; Long, S.; Frugoli, J. The ROOT DETERMINED NODULATION1 gene regulates nodule number in roots of Medicago truncatula and defines a highly conserved, uncharacterized plant gene family. Plant Physiol. 2011, 157, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Ogawa-Ohnishi, M.; Matsushita, W.; Matsubayashi, Y. Identification of three hydroxyproline o-arabinosyltransferases in Arabidopsis thaliana. Nat. Chem. Biol. 2013, 9, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Mortier, V.; Herder, G.; Whitford, R.; van de Velde, W.; Rombauts, S.; D’haeseleer, K.; Hosters, M.; Goormachtig, S. CLE peptides control Medicago truncatula nodulation locally and systemically. Plant Physiol. 2010, 153, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.W.; Lee, Y.W.; Hwang, C.H. Soybean nodule-enhanced CLE peptides in roots act as signals in GmNARK-mediated nodulation suppression. Plant Cell Physiol. 2011, 52, 1613–1627. [Google Scholar] [CrossRef] [PubMed]
- Saur, I.M.L.; Oakes, M.; Djordjevic, M.A.; Imin, N. Crosstalk between the nodulation signaling pathway and the autoregulation of nodulation in Medicago truncatula. New Phytol. 2011, 190, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Mortier, V.; de Wever, E.; Vuylsteke, M.; Holsters, M.; Goormachtig, S. Nodule numbers are governed by interaction between CLE peptides and cytokinin signaling. Plant J. 2012, 70, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, E.; Mukherjee, A.; Smith, L.; Kassaw, T.; Long, S.; Frugoli, J. The lss supernodulation mutant of Medicago truncatula reduces expression of the SUNN gene. Plant Physiol. 2010, 154, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Schultze, M.; Kondorosi, A. Regulation of symbiotic root nodule development. Annu. Rev. Genet. 1998, 32, 33–57. [Google Scholar] [CrossRef] [PubMed]
- Carroll, B.J.; McNeil, D.L.; Gresshoff, P.M. A super-nodulation and nitrate-tolerant symbiotic (nts) soybean mutant. Plant Physiol. 1985, 78, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Caba, J.M.; Recalde, L.; Ligero, F. Nitrate-induced ethylene biosynthesis and the control of nodulation in alfalfa. Plant Cell Environ. 1998, 21, 87–93. [Google Scholar] [CrossRef]
- Baker, B.; Zambryski, P.; Staskawicz, B.; Dinesh-Kumar, S.P. Signaling in plant-microbe interactions. Science 1997, 276, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Singleton, P.W.; van Kessel, C. Effect of localized nitrogen availability to soybean half-root systems on photosynthate partitioning to roots and nodules. Plant Physiol. 1987, 83, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Laguerre, G.; Heulin-Gotty, K.; Brunel, B.; Klonowska, A.; Le Quere, A.; Tillard, P.; Prin, Y.; Cleyet-Marel, J.C.; Lepetit, M. Local and systemic N signaling are involved in Medicago truncatula preference for the most efficient Sinorhizobium symbiotic partners. New Phytol. 2012, 195, 437–449. [Google Scholar]
- Kassaw, T.; Frugoli, J. Simple and efficient methods to generate split roots and grafted plants useful for long-distance signaling studies in Medicago truncatula and other small plants. Plant Methods 2012, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Olsson, J.E.; Nakao, P.; Bohlool, B.B.; Gresshoff, P.M. Lack of systemic supression of nodulation in split root systems of supernodulating soybean (Glycine max [l.] merr.) mutants. Plant Physiol. 1989, 90, 1347–1352. [Google Scholar] [CrossRef]
- Hinson, K. Nodulation responses from nitrogen applied to soybean half-root systems. Agron. J. 1975, 67, 799–804. [Google Scholar] [CrossRef]
- Voisin, A.S.; Munier-Jolain, N.G.; Salon, C. The nodulation process is tightly adjusted to plant growth. An analysis using environmentally and genetically induced variation of nodule number and biomass in pea. Plant Soil. 2010, 337, 399–412. [Google Scholar]
- Jeudy, C.; Ruffel, S.; Freixes, S.; Tillard, P.; Santoni, A.L.; Morel, S.; Journet, E.P.; Duc, G.; Gojon, A.; Lepetit, M.; Salon, C. Adaptation of Medicago truncatula to nitrogen limitation is modulated via local and systemic nodule developmental responses. New Phytol. 2010, 185, 817–828. [Google Scholar]
- Meade, H.M.; Long, S.R.; Ruvkun, G.B.; Brown, S.E.; Ausubel, F.M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J. Bacteriol. 1982, 149, 114–122. [Google Scholar] [PubMed]
- Ruvkun, G.B.; Long, S.R.; Meade, H.M.; van den Bos, R.C.; Ausubel, F.M. Isrm1: A Rhizobium meliloti insertion sequence that transposes preferentially into nitrogen fixation genes. J. Mol. Appl. Genet. 1982, 1, 405–418. [Google Scholar] [PubMed]
- Fisher, R.F.; Egelhoff, T.T.; Mulligan, J.T.; Long, S.R. Specific binding of proteins from Rhizobium meliloti cell-free extracts containing NodD to DNA sequences upstream of inducible nodulation genes. Genes Dev. 1988, 2, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkova, G.E.; Georgiev, G.I. Effect of phosphorus nutrition on the nodulation, nitrogen fixation and nutrient-use efficiency of Bradyrhizobium japonicum_soybean (Glycine max l. Merr.) symbiosis. Bulg. J. Plant Physiol. 2003, 331–335. [Google Scholar]
- Caetano-Anollés, G.; Bauer, W.D. Enhanced nodule initiation on alfalfa by wild-type Rhizobium meliloti co-inoculated with Nod gene mutants and other bacteria. Planta 1988, 174, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Schnabel, E.; Hughes, K.; Frugoli, J. RNAi phenotypes and the localization of a protein::GUS fusion imply a role for Medicago truncatula PIN genes in nodulation. J. Plant Growth Regul. 2006, 25, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Bekki, A.; Trichant, J.C.; Rigaud, J. Nitrogen fixation (C2H2 reduction) by Medicago nodules and bacteroids under sodium chloride stress. Physiol. Plant. 1987, 71, 61–67. [Google Scholar] [CrossRef]
- Duke Environmental Stable Isotope Laboratory. Available online: http://nicholas.duke.edu/devil/ (accessed on 23 April 2015).
- Hualt, E.; Laffont, C.; Wen, J.; Mysore, K.S.; Ratet, P.; Duc, G.; Frugier, F. Local and systemic regulation of plant root system architecture and symbiotic nodulation by a receptor-like kinase. PLOS Genet. 2014, 10, e1004891. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Radzman, N.A.; Binos, S.; Truong, T.T.; Iman, N.; Mariani, M.; Djordjevic, M.A. Novel MtCEP1 peptides produced in vivo differentially regulate root development in Medicago truncatula. J. Exp. Bot. 2015. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassaw, T.; Jr., W.B.; Frugoli, J. Multiple Autoregulation of Nodulation (AON) Signals Identified through Split Root Analysis of Medicago truncatula sunn and rdn1 Mutants. Plants 2015, 4, 209-224. https://0-doi-org.brum.beds.ac.uk/10.3390/plants4020209
Kassaw T, Jr. WB, Frugoli J. Multiple Autoregulation of Nodulation (AON) Signals Identified through Split Root Analysis of Medicago truncatula sunn and rdn1 Mutants. Plants. 2015; 4(2):209-224. https://0-doi-org.brum.beds.ac.uk/10.3390/plants4020209
Chicago/Turabian StyleKassaw, Tessema, William Bridges Jr., and Julia Frugoli. 2015. "Multiple Autoregulation of Nodulation (AON) Signals Identified through Split Root Analysis of Medicago truncatula sunn and rdn1 Mutants" Plants 4, no. 2: 209-224. https://0-doi-org.brum.beds.ac.uk/10.3390/plants4020209