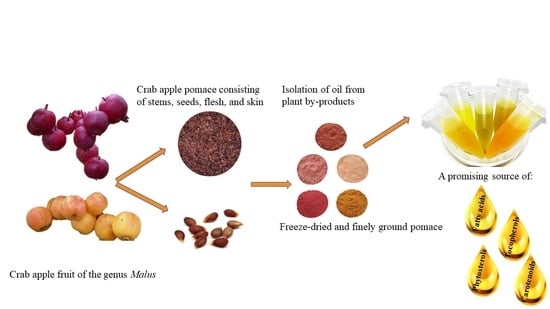
Valorization of Wild Apple (Malus spp.) By-Products as a Source of Essential Fatty Acids, Tocopherols and Phytosterols with Antimicrobial Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. FAMEs of Crab Apple Pomace Oil
2.2. Ts of Crab Apple Pomace Oil
2.3. Phytosterols and Triterpenes of Crab Apple Pomace Oil
2.4. Total Carotenoids of Crab Apple Pomace Oil
2.5. Antioxidant Activity of Crab Apple Pomace Oils
2.6. Antimicrobial Activity of Crab Apple Pomace Oils
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals and Standards
3.3. Soxhlet Extraction
3.4. Preparation of FAs for GC/MS Analysis
3.5. GC Conditions for FAMEs
3.6. GC Conditions for FFAs, Phytosterols, and Triterpenes
3.7. The MS Conditions for FAMEs and Silyl Derivatives Detection
3.8. Standard Compounds Used for Calibration
3.9. Tocopherol Determination Using the RP-HPLC/FLD
3.10. Spectrophotometric Analysis of the Total Carotenoids
3.11. Antioxidant Assays
3.11.1. DPPH• Radical Scavenging Activity
3.11.2. The Ferric Reducing Antioxidant Power (FRAP) Assay
3.12. Minimum Inhibitory Concentration (MIC) Determination
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- FAOSTAT. In: FAO Stat. Database. 2018. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 10 May 2018).
- Statistica. Statistics Portal. 2016. Available online: https://0-www-statista-com.brum.beds.ac.uk/statistics/423198/production-volume-of-apple-juice-eu/ (accessed on 1 May 2018).
- AICV. European Cider Trends. 2014. Available online: http://aicv.org/file.handler?f=CiderTrends2014.pdf (accessed on 10 May 2018).
- Stenmarck, Å.; Jensen, C.; Quested, T.; Moates, G. Estimates of European Food Waste Levels. 2016. Available online: http://www.eu-fusions.org/phocadownload/Publications/Estimates of European food waste levels.pdf (accessed on 15 January 2017).
- Kitrytė, V.; Povilaitis, D.; Kraujalienė, V.; Šulniūtė, V.; Pukalskas, A.; Venskutonis, P.R. Fractionation of sea buckthorn pomace and seeds into valuable components by using high pressure and enzyme-assisted extraction methods. LWT Food Sci. Technol. 2017, 1–5. [Google Scholar] [CrossRef]
- Radenkovs, V.; Juhnevica-Radenkova, K.; Górnaś, P.; Seglina, D. Non-waste technology through the enzymatic hydrolysis of agro-industrial by-products. Trends Food Sci. Technol. 2018, 77, 64–76. [Google Scholar] [CrossRef]
- Gosch, C.; Halbwirth, H.; Kuhn, J.; Miosic, S.; Stich, K. Biosynthesis of phloridzin in apple (Malus domestica Borkh.). Plant Sci. 2009, 176, 223–231. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, Y.; Chen, X.; Wang, Y.; Chen, W.; Xu, Q.; Li, P.; Ma, F. Extraction, identification, and antioxidant and anticancer tests of seven dihydrochalcones from Malus ‘Red Splendor’ fruit. Food Chem. 2017, 231, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Segliņa, D.; Lacis, G.; Pugajeva, I. Dessert and crab apple seeds as a promising and rich source of all four homologues of tocopherol (α, β, γ and δ). LWT Food Sci. Technol. 2014, 59, 211–214. [Google Scholar] [CrossRef]
- Stojiljković, D.; Arsić, I.; Tadić, V. Extracts of wild apple fruit (Malus sylvestris (L.) Mill., Rosaceae) as a source of antioxidant substances for use in production of nutraceuticals and cosmeceuticals. Ind. Crop. Prod. 2016, 80, 165–176. [Google Scholar] [CrossRef]
- Mihailović, N.R.; Mihailović, V.B.; Kreft, S.; Ćirić, A.R.; Joksović, L.G.; Đurđević, P.T. Analysis of phenolics in the peel and pulp of wild apples (Malus sylvestris (L.) Mill.). J. Food Compos. Anal. 2018, 67, 1–9. [Google Scholar] [CrossRef]
- Dadwal, V.; Agrawal, H.; Sonkhla, K.; Joshi, R.; Gupta, M. Characterization of phenolics, amino acids, fatty acids and antioxidant activity in pulp and seeds of high altitude Himalayan crab apple fruits (Malus baccata). J. Food Sci. Technol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Anez-Bustillos, L.; Dao, D.T.; Fell, G.L.; Baker, M.A.; Gura, K.M.; Bistrian, B.R.; Puder, M. Redefining essential fatty acids in the era of novel intravenous lipid emulsions. Clin. Nutr. 2017, 37, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Rudzińska, M.; Segliņa, D. Lipophilic composition of eleven apple seed oils: A promising source of unconventional oil from industry by-products. Ind. Crop. Prod. 2014, 60, 86–91. [Google Scholar] [CrossRef]
- Calpe-Berdiel, L.; Escolà-Gil, J.C.; Blanco-Vaca, F. New insights into the molecular actions of plant sterols and stanols in cholesterol metabolism. Atherosclerosis 2009, 203, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Derdemezis, C.S.; Filippatos, T.D.; Mikhailidis, D.P.; Elisaf, M.S. Effects of plant sterols and stanols beyond low-density lipoprotein cholesterol lowering. J. Cardiovasc. Pharmacol. Ther. 2010, 15, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Llaverias, G.; Escolà-Gil, J.C.; Lerma, E.; Julve, J.; Pons, C.; Cabré, A.; Cofán, M.; Ros, E.; Sánchez-Quesada, J.L.; Blanco-Vaca, F. Phytosterols inhibit the tumor growth and lipoprotein oxidizability induced by a high-fat diet in mice with inherited breast cancer. J. Nutr. Biochem. 2013, 24, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P. Unique variability of tocopherol composition in various seed oils recovered from by-products of apple industry: Rapid and simple determination of all four homologues (α, β, γ and δ) by RP-HPLC/FLD. Food Chem. 2015, 172, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Tuberoso, C.I.G.; Kowalczyk, A.; Sarritzu, E.; Cabras, P. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem. 2007, 103, 1494–1501. [Google Scholar] [CrossRef]
- Walia, M.; Rawat, K.; Bhushan, S.; Padwad, Y.S.; Singh, B. Fatty acid composition, physicochemical properties, antioxidant and cytotoxic activity of apple seed oil obtained from apple pomace. J. Sci. Food Agric. 2014, 94, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antioxidant and antimicrobial properties of dried Portuguese apple variety (Malus domestica Borkh. cv Bravo de Esmolfe). Food Chem. 2018, 240, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Rudzińska, M.; Raczyk, M.; Mišina, I.; Soliven, A.; Lācis, G.; Segliņa, D. Impact of species and variety on concentrations of minor lipophilic bioactive compounds in oils recovered from plum kernels. J. Agric. Food Chem. 2016, 64, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Yi, B.; Kim, M.-J.; Lee, J. Influence of different moisture contents on the stability of tocochromanols in bulk oils at 25 °C storage. J. Am. Oil Chem. Soc. 2018, 197–207. [Google Scholar] [CrossRef]
- Kraujalis, P.; Kraujalienė, V.; Kazernavičiūtė, R.; Venskutonis, P.R. Supercritical carbon dioxide and pressurized liquid extraction of valuable ingredients from Viburnum opulus pomace and berries and evaluation of product characteristics. J. Supercrit. Fluids 2017, 122, 99–108. [Google Scholar] [CrossRef]
- Rodrigues, J.; Miranda, I.; Furquim, L.; Gominho, J.; Vasconcelos, M.; Barradas, G.; Pereira, H.; Bianchi-de-Aguiar, F.; Ferreira-Dias, S. Storage stability of Jatropha curcas L. oil naturally rich in gamma-tocopherol. Ind. Crop. Prod. 2015, 64, 188–193. [Google Scholar] [CrossRef] [Green Version]
- Peschel, S.; Franke, R.; Schreiber, L.; Knoche, M. Composition of the cuticle of developing sweet cherry fruit. Phytochemistry 2007, 68, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Rao, J.; Huber, D.J.; Chang, X.; Xin, F. Wax composition of “Red Fuji” apple fruit during development and during storage after 1-methylcyclopropene treatment. Hortic. Environ. Biotechnol. 2012, 53, 288–297. [Google Scholar] [CrossRef]
- DellaPenna, D.; Mène-Saffrané, L. Vitamin E. In Advances in Botanical Research; Elsevier Science: Burlington, NJ, USA, 2011; Volume 59, pp. 179–227. ISBN 9780123858511. [Google Scholar]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.X.; Lee, M.J.; Liu, A.B.; Yang, Z.; Lin, Y.; Shih, W.J.; Yang, C.S. δ-tocopherol is more active than α- or γ-tocopherol in inhibiting lung tumorigenesis in vivo. Cancer Prev. Res. 2011, 4, 404–413. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.C.; Jorge, N. Bioactive compounds of oils extracted from fruits seeds obtained from agroindustrial waste. Eur. J. Lipid Sci. Technol. 2017, 119, 1–5. [Google Scholar] [CrossRef]
- Katan, M.B.; Grundy, S.M.; Jones, P.; Law, M.; Miettinen, T.; Paoletti, R. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin. Proc. 2003, 78, 965–978. [Google Scholar] [CrossRef]
- Saleem, M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santiago, L.A.; Mayor, A.B.R. Lupeol: An antioxidant triterpene in Ficus pseudopalma Blanco (Moraceae). Asian Pac. J. Trop. Biomed. 2014, 4, 109–118. [Google Scholar] [CrossRef]
- Siddique, H.R.; Saleem, M. Beneficial health effects of lupeol triterpene: A review of preclinical studies. Life Sci. 2011, 88, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.F.G.; Alves, R.E.; Roca, M. Carotenoid composition in oils obtained from palm fruits from the Brazilian Amazon. Grasas y Aceites 2015, 66, e086. [Google Scholar] [CrossRef]
- Fromm, M.; Bayha, S.; Kammerer, D.R.; Carle, R. Identification and quantitation of carotenoids and tocopherols in seed oils recovered from diffrent Rosaceae Species. J. Agric. Food Chem. 2012, 60, 10733–10742. [Google Scholar] [CrossRef] [PubMed]
- Biesalski, H.K.; Böhles, H.; Esterbauer, H.; Fürst, P.; Gey, F.; Hundsdörfer, G.; Kasper, H.; Sies, H.; Weisburger, J. Antioxidant vitamins in prevention. Clin. Nutr. 1997, 16, 151–155. [Google Scholar] [CrossRef]
- Prescha, A.; Grajzer, M.; Dedyk, M.; Grajeta, H. The Antioxidant Activity and Oxidative Stability of Cold-Pressed Oils. J. Am. Oil Chem. Soc. 2014, 91, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, N.; Zu, Y.G.; Fu, Y.J. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008, 108, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Radenkovs, V.; Püssa, T.; Juhnevica-Radenkova, K.; Anton, D.; Seglina, D. Phytochemical characterization and antimicrobial evaluation of young leaf/shoot and press cake extracts from Hippophae rhamnoides L. Food Biosci. 2018, 24, 56–66. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), 5–16. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Guil-Guerrero, J.L.; Ramos, L.; Moreno, C.; Zúñiga-Paredes, J.C.; Carlosama-Yepez, M.; Ruales, P. Antimicrobial activity of plant-food by-products: A review focusing on the tropics. Livest. Sci. 2016, 189, 32–49. [Google Scholar] [CrossRef]
- Mizunaga, S.; Kamiyama, T.; Fukuda, Y.; Takahata, M.; Mitsuyama, J. Influence of inoculum size of Staphylococcus aureus and Pseudomonas aeruginosa on in vitro activities and in vivo efficacy of fluoroquinolones and carbapenems. J. Antimicrob. Chemother. 2005, 56, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Dilika, F.; Bremner, P.D.; Meyer, J.J.M. Antibacterial activity of linoleic and oleic acids isolated from Helichrysum pedunculatum: A plant used during circumcision rites. Fitoterapia 2000, 71, 450–452. [Google Scholar] [CrossRef]
- Juhņeviča-Radenkova, K.; Radenkovs, V. Influence of 1-Methylcyclopropene and ULO conditions on sensory characteristics of apple fruit grown in Latvia. J. Hortic. Res. 2016, 24, 37–46. [Google Scholar] [CrossRef]
- Ruiz, R.P. Gravimetric Measurements of Water. In Handbook of Food Analytical Chemistry, Water, Proteins, Enzymes, Lipids, and Carbohydrates; Wrolstad, R.E., Acree, T.E., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Smith, D., Sporns, P., Eds.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2005; pp. 5–33. ISBN 9780471709084. [Google Scholar]
- Abdolshahi, A.; Majd, M.H.; Rad, J.S.; Taheri, M.; Shabani, A.; Teixeira da Silva, J.A. Choice of solvent extraction technique affects fatty acid composition of pistachio (Pistacia vera L.) oil. J. Food Sci. Technol. 2015, 52, 2422–2427. [Google Scholar] [CrossRef] [PubMed]
- Lelacheur, R.M.; Sonnenberg, L.B.; Singer, P.C.; Christman, R.F.; Charles, M.J. Identification of carbonyl compounds in environmental samples. Environ. Sci. Technol. 1993, 27, 2745–2753. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Górnaś, P.; Siger, A.; Czubinski, J.; Dwiecki, K.; Segliņa, D.; Nogala-Kalucka, M. An alternative RP-HPLC method for the separation and determination of tocopherol and tocotrienol homologues as butter authenticity markers: A comparative study between two European countries. Eur. J. Lipid Sci. Technol. 2014, 116, 895–903. [Google Scholar] [CrossRef]
- Biehler, E.; Mayer, F.; Hoffmann, L.; Krause, E.; Bohn, T. Comparison of 3 spectrophotometric methods for carotenoid determination in frequently consumed fruits and vegetables. J. Food Sci. 2010, 75. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Rao, L.G. Carotenoids and human health (Review). Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, X.; Huang, L. Correlation between antioxidant activities and phenolic contents of radix Angelicae sinensis (Danggui). Molecules 2009, 14, 5349–5361. [Google Scholar] [CrossRef] [PubMed]




| Compound | Retention Index | “Gita” | Malus soulardii | Bernu Prieks | “Ola” | Berzukroga Dzeltenais |
|---|---|---|---|---|---|---|
| FAs | ||||||
| Dodecanoic acid | 1758 | n.d. | 7.2 ± 0.0 a | 7.5 ± 0.0 a | 3.6 ± 0.0 c | 5.3 ± 0.1 b |
| Tetradecanoic acid | 1965 | 9.2 ± 0.0 a | 8.2 ± 0.1 b | 8.1 ± 0.1 b | 3.9 ± 0.0 d | 6.0 ± 0.0 c |
| 9-Oxononanoic acid | 2016 | n.d. | n.d. | n.d. | 4.9 ± 0.0 a | 5.6 ± 0.0 a |
| 9,9-Dimethoxynonanoic acid | 2050 | n.d. | 7.3 ± 0.1 b | 8.9 ± 0.0 a | 5.1 ± 0.0 c | 4.6 ± 0.0 c |
| Nonanedioic acid | 2092 | 9.6 ± 0.0 a | 8.3 ± 0.1 b | 9.1 ± 0.0 ab | n.d. | n.d. |
| Hexadecanoic acid | 2165 | 44.8 ± 0.2 d | 56.8 ± 1.6 c | 71.3 ± 1.3 b | 70.9 ± 1.7 b | 81.5 ± 2.7 a |
| (Z)-7-Hexadecenoic acid | 2186 | n.d. | 7.1 ± 0.2 | n.d. | n.d. | n.d. |
| (Z)-9-Hexadecenoic acid | 2192 | 9.5 ± 0.1 a | 7.2 ± 0.2 b | 7.9 ± 0.4 b | n.d. | n.d. |
| Heptadecanoic acid | 2261 | n.d. | n.d. | n.d. | 3.6 ± 0.0 b | 4.7 ± 0.1 a |
| Octadecanoic acid | 2367 | 18.6 ± 0.1 b | 16.3 ± 0.1 c | 22.2 ± 0.3 a | 14.4 ± 0.2 d | 9.2 ± 0.1 e |
| (Z)-9-Octadecenoic acid | 2385 | 67.1 ± 0.3 e | 95.8 ± 0.4 d | 136.0 ± 2.1 c | 206.9 ± 1.8 a | 164.1 ± 2.1 b |
| 9,10-Dihydroxystearate | 2395 | 1.5 ± 0.0 ac | 1.6 ± 0.1 ac | 1.1 ± 0.0 ab | 0.8 ± 0.0 b | 1.0 ± 0.0 bc |
| (Z,Z)- 9,12-Octadecadienoic acid | 2433 | 149.0 ± 0.2 e | 193.5 ± 4.0 d | 307.8 ± 4.6 c | 468.8 ± 3.1 a | 377.9 ± 2.2 b |
| (Z,Z,Z)-9,12,15-Octadecatrienoic acid | 2496 | 14.6 ± 0.1 c | 19.4 ± 0.4 b | 29.5 ± 0.6 a | 14.6 ± 0.3 c | 9.9 ± 0.0 d |
| Eicosanoic acid | 2571 | 13.7 ± 0.1 a | 9.6 ± 0.0 b | 14.4 ± 0.2 a | 7.9 ± 0.1 c | 5.8 ± 0.0 d |
| (Z)-11-Eicosenoic acid | 2588 | 10.2 ± 0.1 | n.d. | n.d. | n.d. | n.d. |
| Docosanoic acid | 2781 | n.d. | 9.7 ± 0.0 a | 9.6 ± 0.0 a | 5.8 ± 0.0 b | 5.0 ± 0.0 b |
| Total FAs | 346.2 ± 1.2 e | 446.5 ± 7.3 d | 632.3 ± 9.6 c | 810.4 ± 7.2 a | 679.6 ± 7.3 b | |
| Alcohol | ||||||
| Nonacosan-10-ol | 3046 | 6.6 ± 0.1 b | 16.7 ± 0.2 a | 4.9 ± 0.0 c | 3.9 ± 0.1 d | 3.2 ± 0.0 d |
| Ts | ||||||
| δ-Tocopherol | 8.1 | 3.6± 0.1 c | 3.1 ± 0.1 c | 21.5 ± 0.3 a | 4.8 ± 0.1 b | 3.6 ± 0.1 c |
| β-Tocopherol | 9.8 | 2.7 ± 0.1 b | 3.9 ± 0.1 a | 2.9 ± 0.1 b | 2.6 ± 0.1 b | 3.0 ± 0.1 b |
| γ-Tocopherol | 10.3 | 1.1 ± 0.1 d | 2.3 ± 0.1 c | 2.1 ± 0.1 c | 6.8 ± 0.1 a | 4.4 ± 0.1 b |
| α-Tocopherol | 12.0 | 9.4 ± 0.2 c | 13.9 ± 0.3 a | 4.4 ± 0.3 e | 10.2 ± 0.2 b | 7.4 ± 0.2 d |
| Total Ts | 16.8 ± 0.5 f | 23.1 ± 0.6 c | 30.9 ± 0.8 a | 24.4 ± 0.5 d | 18.4 ± 0.5 e |
| Compound | Retention index | “Gita” | Malus soulardii | Bernu Prieks | “Ola” | Berzukroga Dzeltenais |
|---|---|---|---|---|---|---|
| FFAs | ||||||
| Pentadecanoic acid | 1254 | 0.01 ± 0.0 b | 0.1 ± 0.0 a | 0.03 ± 0.0 b | 0.01 ± 0.0 b | 0.04 ± 0.0 b |
| Hexadecanoic acid | 2045 | 3.8 ± 0.1 a | 1.2 ± 0.0 c | 3.2 ± 0.1 ab | 2.6 ± 0.1 b | 3.0 ± 0.0 ab |
| (Z,Z)-9,12-Octadecadienoic acid | 2206 | 2.6 ± 0.1 d | 0.4 ± 0.0 e | 4.0 ± 0.0 c | 14.4 ± 0.1 a | 7.1 ± 0.0 b |
| (Z)-9-Octadecenoic acid | 2212 | 4.2 ± 0.0 c | 0.8 ± 0.0 d | 3.8 ± 0.0 c | 8.3 ± 0.1 a | 5.8 ± 0.1 b |
| (E)-9-Octadecenoic acid | 2220 | 1.1 ± 0.0 a | 0.3 ± 0.0 b | 1.1 ± 0.0 a | 0.5 ± 0.0 b | 0.7 ± 0.0 b |
| Octadecanoic acid | 2239 | 1.3 ± 0.0 ab | 0.3 ± 0.0 c | 1.6 ± 0.0 a | 0.7 ± 0.0 b c | 1.0 ± 0.1 ab |
| Total FFAs | 13.1 ± 0.2 c | 3.0 ± 0.0 d | 13.6 ± 0.1 c | 26.5 ± 0.3 a | 17.6 ± 0.0 b | |
| Glycidyl fatty acid esters, glycerides (GFAE) | ||||||
| 1,2,4-Butanetriol | 1387 | 8.5 ± 0.1 a | 0.1 ± 0.0 b | n.d. | n.d. | n.d. |
| 1-Monopalmitoylglycerol | 2576 | n.d. | n.d. | n.d. | 0.2 ± 0.0 a | 0.01 ± 0.00 b |
| 2-Monolinoleoylglycerol | 2703 | n.d. | n.d. | n.d. | 0.04 ± 0.01 | n.d. |
| 1-Monolinoleoylglycerol | 2732 | 0.4 ± 0.0 b | 0.3 ± 0.0 b | 0.3 ± 0.0 b | 2.0 ± 0.0 a | 0.3± 0.0 b |
| 1-Monooleoylglycerol | 2740 | n.d. | n.d. | n.d. | 0.56 ± 0.0 | n.d. |
| 2-Monostearoylglycerol | 2766 | n.d. | n.d. | n.d. | 0.06 ± 0.0 | n.d. |
| Total GFAE | 8.9 ± 0.1 a | 0.4 ± 0.0c | 0.3 ± 0.0c | 2.9 ± 0.1 b | 0.3 ± 0.0 c | |
| Aliphatic alcohols | ||||||
| 1-Octadecanol | 2155 | 2.6 ± 0.0 a | 0.9 ± 0.0 b | 1.4 ± 0.0 b | n.d. | n.d. |
| 1-Hexacosanol | 2930 | 3.7 ± 0.0 a | 1.7 ± 0.0 b | 1.8 ± 0.0 b | 0.8 ± 0.0 c | 2.0 ± 0.0 b |
| Nonacosan-10-ol | 3046 | 47.8 ± 0.7 a | 37.6 ± 1.5 b | 30.5 ± 0.4 c | 14.5 ± 0.0 d | 14.2 ± 0.1 d |
| 1-Octacosanol | 3127 | 3.5 ± 0.0 a | 1.6 ± 0.0 b | 1.9 ± 0.0 b | 0.8 ± 0.0 c | 1.8 ± 0.0 b |
| Total aliphatic alcohols | 57.5 ± 0.7 a | 41.8 ± 1.5 b | 35.6 ± 0.4 c | 16.1 ± 0.0 e | 18.0 ± 0.1 d | |
| Alkanes | ||||||
| Pentacosane | 2500 | 2.4 ± 0.1 | n.d. | n.d. | n.d. | n.d. |
| Heptacosane | 2700 | 8.2 ± 0.1 a | 1.3 ± 0.1 c | 2.5 ± 0.0 b | 0.81 ± 0.0 d | 1.5 ± 0.0 c |
| Octacosane | 2800 | 2.8 ± 0.0 a | 1.1 ± 0.0b c | 1.6 ± 0.0 b | 0.7 ± 0.0 c | 1.3 ± 0.0 bc |
| Nonacosane | 2900 | 60.6± 0.9 b | 52.1 ± 0.6 c | 61.8 ± 0.3 a | 28.7 ± 0.1 d | 14.9 ± 0.1 e |
| Triacontane | 3000 | n.d. | n.d. | n.d. | 0.6 ± 0.0 | n.d. |
| Hentriacontane | 3100 | n.d. | n.d. | n.d. | 0.7 ± 0.0 | n.d. |
| Total alkanes | 74.1 ± 1.1 a | 54.6 ± 0.7 c | 65.9 ± 0.3 b | 31.6 ± 0.1 d | 17.7 ± 0.1 e | |
| Aldehydes | ||||||
| Triacontanal | 3254 | n.d. | n.d. | n.d. | 4.27 ± 0.0 b | 8.95 ± 0.0 a |
| Compound | Retention Index | “Gita” | Malus soulardii | Bernu Prieks | “Ola” | Berzukroga Dzeltenais |
|---|---|---|---|---|---|---|
| Phytosterols | ||||||
| Campesterol | 3230 | 1.4 ± 0.0 b | 2.5 ± 0.6 a | 0.1 ± 0.0 c | 0.8 ± 0.0 c | 1.7 ± 0.0 b |
| Stigmasterol | 3271 | n.d. | n.d. | 111.0 ± 0.8 a | 0.01 ± 0.0 b | n.d. |
| β-Sitosterol | 3341 | 73.2 ± 0.5 a | 51.5 ± 0.9 c | 13.1 ± 0.7 e | 33.2 ± 0.8 d | 52.8 ± 0.6 b |
| Isofucosterol | 3350 | 2.8 ± 0.0 a | 0.6 ± 0.0 c | n.d. | 1.6 ± 0.0 b | 1.3 ± 0.1 b |
| ∆7-Avenasterol | 3394 | n.d. | n.d. | 0.7 ± 0.0 | n.d. | n.d. |
| Total sterols | 77.4 ± 0.5 b | 54.6 ± 1.5 d | 126.1 ± 1.5 a | 35.6 ± 0.8 e | 55.8 ± 0.7 c | |
| Triterpenes | ||||||
| Squalene | 2794 | n.d. | n.d. | 2.1± 0.0 a | 0.6 ± 0.0 b | 1.3 ± 0.0 b |
| α-Amyrin | 3378 | n.d. | 0.5 ± 0.1 b | 2.5 ± 0.1 a | n.d. | n.d. |
| Lupeol | 3384 | 2.1 ± 0.1 b | 0.05 ± 0.0 e | 0.2 ± 0.0 d | 1.3 ± 0.1 c | 3.0 ± 0.1 a |
| 24-Methylenecycloartanol | 3437 | n.d. | n.d. | 1.4 ± 0.0 | n.d. | n.d. |
| Uvaol | 3510 | 6.6 ± 0.3 a | 0.1 ± 0.0 b | 0.7 ± 0.0 b | 0.2 ± 0.0 b | 0.2 ± 0.0 b |
| Ursolic aldehyde | 3605 | 0.9 ± 0.1 b | n.d. | 2.1 ± 0.0 a | 0.1 ± 0.0 b | 0.02 ± 0.0 c |
| Total triterpenes | 9.6 ± 0.4 a | 0.7 ± 0.0 e | 5.3 ± 0.1 b | 2.2 ± 0.1 d | 4.5 ± 0.1 c | |
| Total carotenoids | 6.4 ± 0.5 d | 12.8 ± 0.7 b | 5.1 ± 0.5 c | 5.4 ± 0.5 c | 14.5 ± 0.6 a | |
| DPPH• assay, mmol TAEC mL−1 | 0.7 ± 0.0 c | 2.4 ± 0.4 a | 0.8 ± 0.1 b | 0.5 ± 0.0 d | 0.7 ± 0.0 c | |
| FRAP assay, mmol TAEC mL−1 | 1.1 ± 0.4 d | 1.0 ± 0.2 d | 2.2 ± 0.2 b | 2.4 ± 0.2 a | 1.9 ± 0.3 c |
| Species | “Gita” | Malus soulardii | Bernu Prieks | “Ola” | Berzukroga Dzeltenais |
|---|---|---|---|---|---|
| Staphylococcus aureus | 62.5 | 62.5 | 62.5 | 31.2 | 62.5 |
| Streptococcus pyogenes | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 |
| Enterococcus faecalis | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 |
| Pseudomonas aeruginosa | 62.5 | 62.5 | 62.5 | 31.2 | 31.2 |
| Escherichia coli | 62.5 | 62.5 | 31.2 | 31.2 | 31.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radenkovs, V.; Kviesis, J.; Juhnevica-Radenkova, K.; Valdovska, A.; Püssa, T.; Klavins, M.; Drudze, I. Valorization of Wild Apple (Malus spp.) By-Products as a Source of Essential Fatty Acids, Tocopherols and Phytosterols with Antimicrobial Activity. Plants 2018, 7, 90. https://0-doi-org.brum.beds.ac.uk/10.3390/plants7040090
Radenkovs V, Kviesis J, Juhnevica-Radenkova K, Valdovska A, Püssa T, Klavins M, Drudze I. Valorization of Wild Apple (Malus spp.) By-Products as a Source of Essential Fatty Acids, Tocopherols and Phytosterols with Antimicrobial Activity. Plants. 2018; 7(4):90. https://0-doi-org.brum.beds.ac.uk/10.3390/plants7040090
Chicago/Turabian StyleRadenkovs, Vitalijs, Jorens Kviesis, Karina Juhnevica-Radenkova, Anda Valdovska, Tõnu Püssa, Maris Klavins, and Inese Drudze. 2018. "Valorization of Wild Apple (Malus spp.) By-Products as a Source of Essential Fatty Acids, Tocopherols and Phytosterols with Antimicrobial Activity" Plants 7, no. 4: 90. https://0-doi-org.brum.beds.ac.uk/10.3390/plants7040090