Biocontrol Potential of Sclerotinia sclerotiorum and Physiological Changes in Soybean in Response to Butia archeri Palm Rhizobacteria
Abstract
:1. Introduction
2. Material and Methods
2.1. Bacterial Isolates and the Phytopathogenic Strain
2.2. Preparation of the Biocontrollers and Inoculation of the Seeds
2.3. Plant Maintenance and Inoculation with the Phytopathogen
2.4. Physiological Analyses
2.5. Experimental Design and Statistical Analyses
3. Results
3.1. Physiological Analyses of Pigments
3.2. Physiological Analyses of Nitrogen Balance, Flavonoid and Anthocyanin Indices
3.3. OJIP Fluorescence Transient Analysis
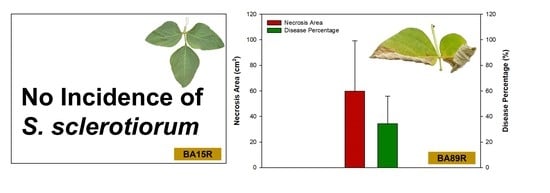
3.4. Incidence of Disease
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Toruño, T.Y.; Stergiopoulos, I.; Coaker, G. Plant-pathogen effectors: Cellular probes interfering with plant defenses in spatial and temporal manners. Annu. Rev. Phytopathol. 2016, 54, 419–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snelders, N.C.; Kettles, G.J.; Rudd, J.J.; Thomma, B.P.H.J. Plant pathogen effector proteins as manipulators of host microbiomes? Mol. Plant Pathol. 2018, 19, 257–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weller, D.M.; Raaijmakers, J.M.; Gardener, B.B.M.; Thomashow, L.S. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu. Rev. Phytopathol. 2004, 40, 309–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhary, D.K.; Johri, B.N. Interactions of Bacillus spp. and plantsx—With special reference to induced systemic resistance (ISR). Microbiol. Res. 2009, 64, 493–513. [Google Scholar] [CrossRef]
- Bakker, P.A.H.M.; Ran, L.X.; Pieterse, C.M.J.; van Loon, L.C. Understanding the involvement of rhizobacteria mediated induction of systemic resistance in biocontrol of plant diseases. Can. J. Plant Pathol. 2003, 25, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Vaz, A.B.M.; Brandão, L.R.; Vieira, M.L.A.; Pimenta, R.S.; Morais, P.B.; Sobral, M.E.G.; Rosa, L.H.; Rosa, C.A. Diversity and antimicrobial activity of fungal endophyte communities associated with plants of Brazilian savanna ecosystems. Afr. J. Microbiol. Res. 2012, 6, 3173–3185. [Google Scholar] [CrossRef]
- Faria, P.S.A.; Senábio, J.A.; Soares, M.A.; Silva, F.G.; Cunha, A.P.A.; Souchie, E.L. Assessment of functional traits in the assemblage of endophytic fungi of Anacardium othonianum Rizzini. Pak. J. Bot. 2016, 48, 1241–1252. [Google Scholar]
- Chagas, M.B.O.; Santos, I.P.; Silva, L.C.N.; Correia, M.T.S.; Araújo, J.M.; Cavalcanti, M.S.; Lima, V.L.M. Antimicrobial activity of cultivable endophytic fungi associated with Hancornia Speciosa Gomes Bark. Open Microbiol. J. 2017, 11, 179–188. [Google Scholar] [CrossRef]
- Silva, C.F.; Senabio, J.A.; Pinheiro, L.C.; Soares, M.A.; Souchie, E.L. Isolation and genetic characterization of endophytic and rhizospheric microorganisms from Butia purpurascens Glassman. Afr. J. Microbiol. Res. 2015, 9, 1907–1916. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.F.; Vitorino, L.C.; Soares, M.A.; Souchie, E.L. Multifunctional potential of endophytic and rhizospheric microbial isolates associated with Butia purpurascens roots for promoting plant growth. Antonie van Leeuwenhoek 2018, 111, 2157–2174. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.S.; Felfili, J.M.; Marimon, B.S.; Scariot, A. Diversidade, estrutura e distribuição espacial de palmeiras em um cerrado sensu stricto no Brasil Central—DF. Rev. Bras. Bot. 2003, 26, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Soares, K.P. Le genre Butia. Principes 2015, 1, 12–57. [Google Scholar]
- Mamede, M.C.H.; Souza, V.; Prado, J.; Barros, F.; Wanderley, M.G.L.; Rando, J.G. Livro Vermelho Das Espécies Vegetais Ameaçadas do Estado de São Paulo, 1st ed.; Instituto de Botânica: São Paulo, Brazil, 2007. [Google Scholar]
- Vitorino, L.C.; Palharini, K.M.Z.; Rocha, A.F.S.R.; Prates, L.S.; Goulart, L.G.; Silva, A.L.; Bessa, L.A. Application of bacteria symbiotic with Butia archeri (Arecaceae) to the biocontrol of the phytopathogenic fungi that deteriorate seeds of Glycine max. Seed Sci. Technol. 2019, 47, 325–341. [Google Scholar] [CrossRef]
- Ranjan, A.; Jayaraman, D.; Grau, C.; Whitham, S.A.; Ané, J.M.; Smith, D.L.; Kabbage, M. The pathogenic development of Sclerotinia sclerotiorum in soybean requires specific host NADPH oxidases. Mol. Plant Pathol. 2018, 19, 700–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willbur, J.; McCaghey, M.; Kabbage, M.; Smith, D.L. An overview of the Sclerotinia sclerotiorum pathosystem in soybean: Impact, fungal biology, and current management strategies. Trop. Plant Pathol. 2019, 44, 3–11. [Google Scholar] [CrossRef]
- Hoffman, D.D.; Hartman, G.L.; Mueller, D.S.; Leitz, R.A.; Nickell, C.D.; Pedersen, W.L. Yield and seed quality of soybean cultivars infected with Sclerotinia sclerotiorum. Plant Dis. 1998, 82, 826–829. [Google Scholar] [CrossRef] [Green Version]
- Danielson, G.A.; Nelson, B.D.; Helms, T.C. Effect of Sclerotinia stem rot on yield of soybean inoculated at different growth stages. Plant Dis. 2004, 88, 297–300. [Google Scholar] [CrossRef]
- Venturoso, L.R.; Walber, L.M.A.B.; Gavassonil, L.; Venturoso, L.A.C.; Pontim, B.C.A.; Reis, G.F. Inoculation of Sclerotinia sclerotiorum in seed of oleaginous plants: Transmission and effects on emergence. Cienc. Rural 2015, 45, 788–793. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Xiao, J.; Zhu, W.; Yang, Y.; Mei, J.; Bi, C.; Quian, W.; Qing, L.; Tan, W. Ss-Rhs1, a secretory Rhs repeat-containing protein, is required for the virulence of Sclerotinia sclerotiorum. Mol. Plant Pathol. 2017, 18, 1052–1061. [Google Scholar] [CrossRef]
- Allen, T.W.; Bradley, C.A.; Sisson, A.J.; Byamukama, E.; Chilvers, M.I.; Coker, C.M.; Collins, A.A.; Damicone, J.P.; Dorrance, A.E.; Dufault, N.S.; et al. Soybean yield loss estimates due to diseases in the United States and Ontario, Canada from 2010 to 2014. Plant Health Prog. 2017, 18, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Koenning, S.R.; Wrather, J.A. Suppression of soybean yield potential in the continental United States by plant diseases from 2006 to 2009. Plant Health Prog. 2010, 11. [Google Scholar] [CrossRef] [Green Version]
- United States Department of Agriculture—National Agricultural Statistics Service (USDA-NASS). United States Soybean Prices; USDA-NASS: Washington, DC, USA, 2017.
- Cunha, W.G.; Tinoco, M.L.P.; Pancoti, H.L.; Ribeiro, R.E.; Aragão, F.J.L. High resistance to Sclerotinia sclerotiorum in transgenic soybean plants transformed to express an oxalate decarboxylase gene. Plant Pathol. 2010, 59, 654–660. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.; Chapman, S.; Jensen, B. Wisconsin Field Crops Pathology Fungicide Tests Summary. UW Field Crop Pathology. 2014. Available online: http://fyi.uwex.edu/fieldcroppathology/research-summaries/ (accessed on 10 January 2019).
- Huzar-Novakowiski, J.; Paul, P.A.; Dorrance, A.E. Host resistance and chemical control for management of Sclerotinia stem rot of soybean in Ohio. Phytophatology 2017, 107, 937–949. [Google Scholar] [CrossRef] [Green Version]
- Sumida, C.H.; Daniel, J.F.S.; Araujod, A.P.C.S.; Peitl, D.C.; Abreu, L.M.; Dekker, R.F.H.; Canteri, M.G. Trichoderma asperelloides antagonism to nine Sclerotinia sclerotiorum strains and biological control of white mold disease in soybean plants. Biocontrol Sci. Technol. 2018, 28, 142–156. [Google Scholar] [CrossRef]
- Elsheshtawi, M.; Elkhaky, M.T.; Sayed, S.R.; Bahkali, A.H.; Mohammed, A.A.; Gambhir, D.; Mansur, A.S.; Elgorban, A.M. Integrated control of white rot disease on beans caused by Sclerotinia sclerotiorum using Contans® and reduced fungicides application. Saudi J. Biol. Sci. 2017, 24, 405–409. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Ge, H.; Zhang, F.; Guo, N.; Wang, Y.; Chen, L.; Ji, X.; Li, C. Biocontrol potential of Trichoderma harzianum isolate T-aloe against Sclerotinia sclerotiorum in soybean. Plant Physiol. Biochem. 2016, 100, 64–74. [Google Scholar] [CrossRef]
- Jones, E.E.; Bienkowski, D.A.; Stewart, A. The importance of water potential range tolerance as a limiting factor on Trichoderma spp. biocontrol of Sclerotinia sclerotiorum. Ann. Appl. Biol. 2016, 168, 41–51. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Z.; Gao, H.; Liu, M.; Fan, X. Mechanisms by which the infection of Sclerotinia sclerotiorum (Lib.) de Bary affects the photosynthetic performance in tobacco leaves. BMC Plant Biol. 2014, 14, 240. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, D.; Fan, L.; McRae, K.; Walker, B.; MacKay, R.; Doucette, C. Effects of ozone treatment on Botrytis cinerea and Sclerotinia sclerotiorum in relation to horticultural product quality. J. Food Sci. 2009, 74, M250–M257. [Google Scholar] [CrossRef]
- JianWen, B.; GuAng, Y.; Hui-Yuan, G.; Yu-Jiao, J.; Li-Tao, Z.; Dan-Dan, C.; Xin, W. Inhibition mechanism of photosynthesis in cucumber leaves infected by Sclerotinia sclerotiorum (Lib.) de Bary. Acta Phytopathol. Sin. 2009, 39, 613–621. [Google Scholar]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanism, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor and Francis: New York, NY, USA, 2000; pp. 445–483. [Google Scholar]
- Ferreira, T.; Rasband, W. O ImageJ Guia do Usuário—Version 1.43. 2010. Available online: http://rsbweb.nih.gov/ij/docs/user-guide.pdf (accessed on 20 February 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 31 January 2018).
- Saraf, M.; Thakker, A.; Patel, B.V. Biocontrol activity of different species of Pseudomonas against phytopathogenic fungi in vivo and in vitro conditions. Int. J. Biotechnol. Biochem. 2008, 4, 223. [Google Scholar]
- Munjal, V.; Nadakkakath, A.V.; Sheoran, N.; Kundu, A.; Venugopal, V.; Subaharan, K.; Rajamma, S.; Eapen, S.J.; Kumar, A. Genotyping and identification of broad spectrum antimicrobial volatiles in black pepper root endophytic biocontrol agent, Bacillus megaterium BP17. Biol. Control 2016, 92, 66–76. [Google Scholar] [CrossRef]
- Saeid, A.; Prochownik, E.; Dobrowolska-Iwanek, J. Phosphorus solubilization by Bacillus species. Molecules 2018, 23, 2897. [Google Scholar] [CrossRef] [Green Version]
- Do Carmo, T.S.; Moreira, F.S.; Cabral, B.V.; Dantas, R.C.C.; de Resende, M.M.; Cardoso, V.L.; Ribeiro, E.J. Phosphorus recovery from phosphate rocks using phosphate-solubilizing bacteria. Geomicrobiol. J. 2019, 36, 195–203. [Google Scholar] [CrossRef]
- Wagi, S.; Ahmed, A. Bacillus spp.: Potent microfactories of bacterial IAA. PeerJ 2019, 7, e7258. [Google Scholar] [CrossRef] [Green Version]
- Sansinenea, E. Bacillus spp.: As plant growth-promoting bacteria. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms; Singh, H., Keswani, C., Reddy, M., Sansinenea, E., García-Estrada, C., Eds.; Springer: Singapore, 2019; pp. 225–237. [Google Scholar]
- Han, Q.Q.; Lü, X.P.; Bai, J.P.; Qiao, Y.; Paré, P.W.; Wang, S.M.; Zhang, J.L.; Wu, Y.N.; Pang, X.P.; Xu, W.B.; et al. Beneficial soil bacterium Bacillus subtilis (GB03) augments salt tolerance of white clover. Front. Plant Sci. 2014, 5, 525. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Gillmeister, M.; Ballert, S.; Raschke, A.; Geistlinger, J.; Kabrodt, K.; Baltruschat, H.; Deising, H.B.; Schellenberg, I. Polyphenols from rheum roots inhibit growth of fungal and oomycete phytopathogens and induce plant disease resistance. Plant Dis. 2019, 103, 1674–1684. [Google Scholar] [CrossRef]
- Ammar, M.; Nenaah, G.E.; Mohamed, A.H.H. Antifungal activity of prenylated flavonoids isolated from Tephrosia apollinea L. against four phytopathogenic fungi. Crop Prot. 2013, 49, 21–25. [Google Scholar] [CrossRef]
- Fraternale, D.; Ricci, D.; Verardo, G.; Gorassini, A.; Stocchi, V.; Sestili, P. Activity of Vitis vinifera tendrils extract against phytopathogenic fungi. Nat. Prod. Commun. 2015, 10, 1037–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolova, M.T.; Yordanov, P.; Slavov, S.; Berkov, S. Antifungal activity of plant extracts against phytopathogenic fungi. J. BioSci. Biotechnol. 2017, 6, 155–161. [Google Scholar]
- Jiménez-Reyes, M.F.; Carrasco, H.; Olea, A.; Silva-Moreno, E. Natural compounds: A sustainable alternative for controlling phytopathogens. PeerJ Preprints 2018, 6, e26664v1. [Google Scholar] [CrossRef]
- Baghbani, F.; Lotfi, R.; Moharramnejad, S.; Bandehagh, A.; Roostaei, M.; Rastogi, A.; Kalaji, H.M. Impact of Fusarium verticillioides on chlorophyll fluorescence parameters of two maize lines. Eur. J. Plant Pathol. 2018, 154, 337–356. [Google Scholar] [CrossRef] [Green Version]
- Zhori, A.; Meco, M.; Brandl, H.; Bachofen, R. In situ chlorophyll fluorescence kinetics as a tool to quantify effects on photosynthesis in Euphorbia cyparissias by a parasitic infection of the rust fungus Uromyces pisi. BMC Res. Notes 2015, 8, 698. [Google Scholar] [CrossRef] [Green Version]
- Bassanezi, R.B.; Amorin, M.; Bergamin Filho, A.; Berger, R.D. Gas exchange and emission of chlorophyll fluorescence during the monocycle of rust, angular leaf spot and anthracnose on bean leaves as a function of their trophic characteristics. J. Phytopathol. 2002, 150, 37–47. [Google Scholar] [CrossRef]
- Martins, J.P.R.; Schimildt, E.R.; Alexandre, R.S.; Falqueto, A.R.; Otoni, W.C. Chlorophyll a fluorescence and growth of Neoregelia concentrica (Bromeliaceae) during acclimatization in response to light levels. In Vitro Cell Dev. 2015, 51, 471–481. [Google Scholar] [CrossRef]
- Yusuf, M.M.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Govindjee Sarin, N.B. Overexpression of γ-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: Physiological and chlorophyll fluorescence measurements. Biochim. Biophys. Acta 2010, 1797, 1428–1438. [Google Scholar] [CrossRef] [Green Version]
- Rosa, W.S.; Martins, J.P.R.; Rodrigues, E.S.; de Almeida Rodrigues, L.C.; Gontijo, A.B.P.L.; Falqueto, A.R. Photosynthetic apparatus performance in function of the cytokinins used during the in vitro multiplication of Aechmea blanchetiana (Bromeliaceae). Plant Cell Tissue Organ. Cult. 2018, 133, 339–350. [Google Scholar] [CrossRef]
- Moradi, N.; Rahimian, H.; Dastani, A.; Babaiezad; Yaqoubian, Y. Screening of resistant to powdered cucumber cultivars and comparison of chlorophyll fluorescence in sensitive and resistant cultivars. J. Plant Prot. 2017, 31, 466–474. [Google Scholar]
- Xie, S.; Jiang, H.; Ding, T.; Xu, Q.; Xai, W.; Cheng, B. Bacillus amyloliquefaciens FZB42 represses plant miR846 to induce systemic resistance via a jasmonic acid-dependent signalling pathway. Mol. Plant Pathol. 2018, 19, 1612–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, S.; Surovy, M.Z.; Gupta, D.R.; Mahmud, N.U.; Chanclud, E.; Win, J.; Kamoun, S.; Islam, T. Genomic analyses reveal that biocontrol of wheat blast by Bacillus spp. may be linked with production of antimicrobial compounds and induced systemic resistance in host plants. Figshare 2018, 17, 48. [Google Scholar]
- Figueroa-López, A.M.; Cordero-Ramírez, J.D.; Martínez-Álvarez, J.C.; López-Meyer, M.; Lizárraga-Sánchez, G.J.; Félix-Gastélum, R.; Castro-Martínez, C.; Maldonado-Mendoza, I.E. Rhizospheric bacteria of maize with potential for biocontrol of Fusarium verticillioides. SpringerPlus 2016, 5, 330. [Google Scholar] [CrossRef] [Green Version]
- Díaz Herrera, S.D.; Grossi, C.; Zawoznik, M.; Groppa, M.D. Wheat seeds harbour bacterial endophytes with potential as plant growth promoters and biocontrol agents of Fusarium graminearum. Microbiol. Res. 2016, 186, 37–43. [Google Scholar] [CrossRef]
- Sangiogo, M.; Rodriguez, D.P.; Moccellin, R.; Bermudez, J.M.M.; Corrêa, B.O.; Moura, A.B. Foliar spraying with bacterial biocontrol agents for the control of common bacterial blight of bean. Pesq. Agropec. Bras. 2018, 53, 1101–1108. [Google Scholar] [CrossRef]
- Gupta, V.; Buch, A. Pseudomonas aeruginosa predominates as multifaceted rhizospheric bacteria with combined abilities of P-solubilization and biocontrol. J. Pure Appl. Microbiol. 2019, 13, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Chenniappan, C.; Narayanasamy, M.; Daniel, G.M.; Ramaraj, G.B.; Ponnusamy, P.; Sekar, J.; Ramalingam, P.V. Biocontrol efficiency of native plant growth promoting rhizobacteria against rhizome rot disease of turmeric. Biol. Control 2019, 129, 55–64. [Google Scholar] [CrossRef]
- Roberts, D.P.; Short, N.M.J.; Maloney, A.P.; Nelson, E.B.; Schaff, D.A. Role of colonization in biocontrol: Studies with Enterobacter cloacae. Plant Sci. 1994, 101, 83–89. [Google Scholar] [CrossRef]
- Etesami, H.; Alikhani, H.A. Bacillus species as the most promising bacterial biocontrol agents in rhizosphere and endorhiza of plants grown in rotation with each other. Eur. J. Plant Pathol. 2018, 150, 497–506. [Google Scholar] [CrossRef]
- Sun, G.; Yao, T.; Feng, C.; Chen, L.; Li, J.; Wang, L. Identification and biocontrol potential of antagonistic bacteria strains against Sclerotinia sclerotiorum and their growth-promoting effects on Brassica napus. Biol. Control 2017, 104, 35–43. [Google Scholar] [CrossRef]
- Mahartha, K.A.; Suprapta, D.N. Efficacy of Enterobacter cloacae KtB3 to control damping-off disease on soybean caused by Sclerotium rolfsii. Int. J. Agric. Biol. 2018, 20, 871–876. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.K.; Sankaralingam, A. Efficacy of biocontrol agents in the management of head rot of cabbage (Brassica oleracea var. capitata) caused by Sclerotinia sclerotiorum. Afr. J. Microbiol. Res 2016, 10, 1711–1714. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.E.; Hossain, D.M.; Suzuki, K.; Shiiya, A.; Suzuki, K.; Dey, T.K.; Nonaka, M.; Harada, N. Suppressive effects of Bacillus spp. on mycelia, apothecia and sclerotia formation of Sclerotinia sclerotiorum and potential as biological control of white mold on mustard. Aust. Plant Pathol. 2016, 45, 103–117. [Google Scholar] [CrossRef]
- Lozano, G.L.; Holt, J.; Ravel, J.; Rasko, D.A.; Thomas, M.G.; Handelsman, J. Draft genome sequence of biocontrol agent Bacillus cereus UW85. Genome Announc. 2016, 4, e00910-16. [Google Scholar] [CrossRef] [Green Version]
- Taghavi, S.; van der Lelie, D.; Hoffman, A.; Zhang, Y.B.; Walla, M.D.; Vangronsveld, J.; Newman, L.; Monchy, S. Genome sequence of the plant growth promoting endophytic bacterium Enterobacter sp. 638. PLoS Genet. 2010, 6, e1000943. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Wang, L.; Zhu, K.; Hou, S.; Chen, L.; Mi, D.; Gui, Y.; Qi, Y.; Jiang, C.; Guo, J.-H. Plant root exudates are involved in Bacillus cereus AR156 mediated biocontrol against Ralstonia solanacearum. Front. Microbiol. 2019, 10, 98. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.H.; Fan, Z.H.; Xie, P.; Guo, J.H. Bacillus cereus AR156 extracellular polysaccharides served as a novel micro-associated molecular pattern to induced systemic immunity to Pst DC3000 in Arabidopsis. Front. Microbiol. 2016, 7, 664. [Google Scholar] [CrossRef] [Green Version]






| Treatment | Pre-Pathogen | Post-Pathogen | ||||
|---|---|---|---|---|---|---|
| Chlorophyll a | Chlorophyll b | Chlorophyll Total | Chlorophyll a | Chlorophyll b | Chlorophyll Total | |
| Pseudomonas putida (BA15R) | 25.73 ± 0.74 * Ca | 8.40 ± 0.48 Aa | 34.13 ± 1.19 Ba | 24.30 ± 1.28 Ba | 6.22 ± 0.60 Bb | 30.52 ± 1.88 Ba |
| Bacillus pumilus (BA25R) | 26.10 ± 0.99 Ca | 8.58 ± 0.58 Aa | 34.68 ± 1.47 Ba | 23.95 ± 0.64 Bb | 5.82 ± 0.22 Cb | 29.88 ± 0.76 Bb |
| Pantoea cypripedii (BA45R) | 22.05 ± 1.84 Ca | 6.15 ± 0.87 Aa | 28.20 ± 0.67 Ba | 22.57 ± 0.45 Ba | 6.67 ± 0.24 Ba | 29.24 ± 0.67 Ba |
| Enterobacter sp. (BA48R) | 25.40 ± 1.08 Ca | 8.08 ± 0.36 Aa | 33.78 ± 1.36 Ba | 21.88 ± 1.60 Cb | 5.05 ± 0.60 Cb | 26.93 ± 2.19 Cb |
| Bacillus cereus (BA78R) | 24.67 ± 1.19 Ca | 7.37 ± 0.27 Aa | 32.03 ± 1.42 Ba | 21.07 ± 0.79 Cb | 5.02 ± 0.39 Cb | 26.08 ± 1.15 Cb |
| Enterobacter sp. (BA80R) | 29.30 ± 0.86 Ba | 9.07 ± 0.73 Aa | 38.37 ± 1.54 Aa | 26.21 0.64 Ab | 6.42 ± 0.31 Bb | 32.63 ± 0.95 Ab |
| Bacillus cereus (BA81R) | 30.63 ± 0.95 Aa | 9.85 ± 0.55 Aa | 40.48 ± 1.47 Aa | 26.93 ± 1.14 Ab | 7.42 ± 0.55 Ab | 34.02 ± 1.84 Ab |
| Bacillus cereus (BA88R) | 24.80 ± 1.18 Ca | 7.62 ± 0.37 Aa | 32.42 ± 1.60 Ba | 26.39 ± 0.66 Aa | 6.92 ± 0.13 Ba | 33.35 ± 0.76 Aa |
| Brevibacillus brevis (BA89R) | 24.42 ± 0.62 Ca | 8.00 ± 0.26 Aa | 32.42 ± 0.83 Ba | 25.77 ± 2.59 Ba | 5.61 ± 1.49 Cb | 31.38 ± 4.07 Ba |
| Enterobacter sp. (BA103R) | 28.35 ± 0.80 Ba | 9.07 ± 0.35 Aa | 37.42 ± 0.96 Aa | 24.53 ± 1.87 Bb | 6.22 ± 0.66 Bb | 30.75 ± 2.51 Bb |
| Enterobacter oryzae (BA106R) | 24.97 ± 0.80 Ca | 7.38 ± 0.27 Aa | 32.35 ± 0.84 Ba | 24.18 ± 1.79 Ba | 6.70 ± 0.66 Ba | 30.50 ± 2.56 Bb |
| Enterobacter sp. (BA110R) | 27.37 ± 1.17 Ba | 8.15 ± 0.75 Aa | 35.52 ± 1.81 Ba | 26.72 ± 1.20 Aa | 7.35 ± 0.57 Aa | 34.38 ± 1.71 Ab |
| Bacillus sp. (BA122R) | 25.68 ± 0.78 Ca | 8.15 ± 0.60 Aa | 33.83 ± 1.32 Ba | 27.03 ± 0.73 Aa | 7.68 ± 0.28 Aa | 34.72 ± 0.97 Aa |
| Enterobacter asburiae (BA123R) | 25.12 ± 1.16 Ca | 7.68 ± 0.59 Aa | 32.13 ± 2.09 Ba | 26.55 ± 0.58 Aa | 6.73 ± 0.36 Ba | 33.25 ± 0.90 Aa |
| Enterobacter asburiae (BA203R) | 25.52 ± 1.21 Ca | 8.18 ± 0.61 Aa | 33.70 ± 1.75 Ba | 23.77 ± 0.82 Ba | 6.33 ± 0.56 Bb | 30.60 ± 1.68 Bb |
| Control | 29.08 ± 0.92 Ba | 9.92 ± 0.31 Aa | 39.02 ± 1.15 Aa | 21.67 ± 1.23 Bb | 5.27 ± 0.44 Cb | 26.93 ± 1.67 Bb |
| Treatment | Pre-Pathogen | Post-Pathogen | ||||
|---|---|---|---|---|---|---|
| NBI | FLAV | ANTH | NBI | FLAV | ANTH | |
| Pseudomonas putida (BA15R) | 21.83 ± 0.14 * Ba | 1.23 ± 0.03 Ab | 0.24 ± 0.01 Ab | 13.50 ± 3.26 Bb | 1.56 ± 0.06 Aa | 0.32 ± 0.04 Aa |
| Bacillus pumilus (BA25R) | 21.90 ± 1.74 Ba | 1.21 ± 0.08 Ab | 0.28 ± 0.03 Aa | 13.13 ± 1.98 Bb | 1.56 ± 0.08 Aa | 0.30 ± 0.01 Aa |
| Pantoea cypripedii (BA45R) | 19.53 ± 1.48 Ba | 1.30 ± 0.07 Ab | 0.27 ± 0.03 Ab | 14.13 ± 0.30 Bb | 1.49 ± 0.05 Ba | 0.31 ± 0.01 Aa |
| Enterobacter sp. (BA48R) | 18.83 ± 1.00 Ba | 1.30 ± 0.06 Ab | 0.25 ± 0.01 Ab | 13.03 ± 2.98 Bb | 1.52 ± 0.02 Ba | 0.32 ± 0.03 Aa |
| Bacillus cereus (BA78R) | 21.17 ± 1.90 Ba | 1.23 ± 0.09 Ab | 0.23 ± 0.01 Ab | 13.30 ± 0.25 Bb | 1.40 ± 0.10 Ba | 0.33 ± 0.01 Aa |
| Enterobacter sp. (BA80R) | 23.87 ± 0.81 Ba | 1.16 ± 0.04 Bb | 0.26 ± 0.01 Ab | 14.43 ± 2.82 Bb | 1.53 ± 0.13 Ba | 0.32 ± 0.03 Aa |
| Bacillus cereus (BA81R) | 27.83 ± 2.94 Aa | 1.13 ± 0.10 Bb | 0.22 ± 0.00 Ab | 14.63 ± 0.92 Bb | 1.43 ± 0.06 Ba | 0.31 ± 0.01 Aa |
| Bacillus cereus (BA88R) | 23.30 ± 1.72 Ba | 1.20 ± 0.05 Ab | 0.27 ± 0.01 Ab | 18.87 ± 2.87 Ab | 1.39 ± 0.28 Ba | 0.29 ± 0.02 Ba |
| Brevibacillus brevis (BA89R) | 19.87 ± 1.22 Ba | 1.23 ± 0.03 Ab | 0.23 ± 0.01 Ab | 15.20 ± 3.80 Bb | 1.44 ± 0.11 Ba | 0.31 ± 0.04 Aa |
| Enterobacter sp. (BA103R) | 29.10 ± 0.81 Aa | 1.03 ±0.03 Bb | 0.24 ± 0.01 Ab | 15.83 ± 2.80 Bb | 1.39 ± 0.07 Ba | 0.31 ± 0.03 Aa |
| Enterobacter oryzae (BA106R) | 28.90 ± 1.71 Aa | 1.03 ±0.06 Ba | 0.26 ± 0.01 Ab | 13.27 ± 1.51 Bb | 1.08 ± 0.06 Ba | 0.31 ± 0.02 Aa |
| Enterobacter sp. (BA110R) | 23.73 ± 1.50 Ba | 1.23 ± 0.07 Ab | 0.25 ± 0.03 Ab | 15.60 ± 1.11 Bb | 1.39 ± 0.08 Ba | 0.30 ± 0.00 Aa |
| Bacillus sp. (BA122R) | 24.13 ± 1.49 Ba | 1.18 ± 0.03 Ab | 0.24 ± 0.01 Ab | 14.73 ± 2.29 Bb | 1.45 ± 0.10 Ba | 0.30 ± 0.02 Aa |
| Enterobacter asburiae (BA123R) | 24.40 ±1.32 Ba | 1.21 ± 0.06 Ab | 0.23 ± 0.00 Ab | 15.87 ± 2.01 Bb | 1.57 ± 0.06 Aa | 0.28 ± 0.02 Ba |
| Enterobacter asburiae (BA203R) | 24.00 ± 0.17 Ba | 1.21 ± 0.04 Ab | 0.24 ± 0.02 Ab | 14.60 ± 1.81 Bb | 1.46 ± 0.08 Ba | 0.31 ± 0.02 Aa |
| Control | 23.03 ± 1.77 Ba | 1.25 ± 0.06 Ab | 0.22 ± 0.01 Ab | 14.00 ± 0.85 Bb | 1.48 ± 0.05 Ba | 0.29 ± 0.02 Ba |
| Treatment | Pre-Pathogen | Post-Pathogen | ||||||
|---|---|---|---|---|---|---|---|---|
| FV/FM | PIABS | Di0/RC | PITOTAL | FV/FM | PIABS | Di0/RC | PITOTAL | |
| Pseudomonas putida (BA15R) | 0.801 ± 0.000 * Aa | 1.558 ± 0.085 Ba | 0.549 ± 0.016 Cb | 1.810 ± 0.053 Aa | 0.749 ± 0.015 Bb | 0.540 ± 0.078 Cb | 0.759 ± 0.059 Ba | 0.770 ± 0.005 Bb |
| Bacillus pumilus (BA25R) | 0.805 ± 0.008 Aa | 1.635 ± 0.200 Ba | 0.542 ± 0.038 Cb | 1.720 ± 0.138 Aa | 0.750 ± 0.009 Bb | 0.540 ± 0.034 Cb | 0.762 ± 0.033 Ba | 0.880 ± 0.043 Bb |
| Pantoea cypripedii (BA45R) | 0.794 ± 0.001 Aa | 1.229 ± 0.106 Ca | 0.579 ± 0.012 Ca | 1.540 ± 0.155 Aa | 0.762 ± 0.033 Ba | 0.764 ± 0.327 Bb | 0.701 ± 0.153 Ba | 0.100 ± 0.413 Cb |
| Enterobacter sp. (BA48R) | 0.767 ± 0.004 Ca | 0.889 ± 0.097 Da | 0.720 ± 0.020 Aa | 1.660 ± 0.167 Aa | 0.768 ± 0.014 Ba | 0.793 ± 0.221 Ba | 0.600 ± 0.052 Cb | 1.280 ± 0.107 Ab |
| Bacillus cereus (BA78R) | 0.755 ± 0.003 Da | 0.694 ± 0.093 Da | 0.764 ± 0.033 Aa | 2.040 ± 0.408 Aa | 0.758 ± 0.026 Ba | 0.871 ± 0.225 Ba | 0.755 ± 0.129 Ba | 1.240 ± 0.080 Ab |
| Enterobacter sp. (BA80R) | 0.798 ± 0.001 Ab | 1.42 ± 0.041 Ba | 0.549 ± 0.009 Ca | 2.060 ± 0.077 Aa | 0.807 ± 0.001 Aa | 1.321 ± 0.144 Aa | 0.500 ± 0.012 Cb | 1.190 ± 0.097 Ab |
| Bacillus cereus (BA81R) | 0.807 ± 0.001 Aa | 1.621 ± 0.085 Ba | 0.501 ± 0.010 Cb | 2.240 ± 0.050 Aa | 0.741 ± 0.014 Bb | 0.478 ± 0.062 Cb | 0.787 ± 0.078 Ba | 1.100 ± 0.191 Ab |
| Bacillus cereus (BA88R) | 0.787 ± 0.001 Ba | 1.168 ± 0.023 Ca | 0.623 ± 0.011 Ba | 2.000 ± 0.157 Aa | 0.785 ± 0.006 Aa | 1.111 ± 0.028 Aa | 0.602 ± 0.012 Cb | 1.300 ± 0.028 Ab |
| Brevibacillus brevis (BA89R) | 0.786 ± 0.002 Ba | 1.231 ± 0.034 Ca | 0.596 ± 0.006 Ba | 2.750 ± 0.510 Aa | 0.784 ± 0.006 Aa | 0.916 ± 0.105 Ba | 0.594 ± 0.034 Ca | 1.200 ± 0.094 Ab |
| Enterobacter sp. (BA103R) | 0.801 ± 0.006 Aa | 1.467 ± 0.061 Ba | 0.553 ± 0.021 Ca | 1.750 ± 0.069 Aa | 0.804 ± 0.004 Aa | 1.355 ± 0.067 Aa | 0.500 ± 0.017 Cb | 1.020 ± 0.017 Ab |
| Enterobacter oryzae (BA106R) | 0.816 ± 0.001 Aa | 2.149 ± 0.057 Aa | 0.472 ± 0.008 Ca | 2.620 ± 0.081 Aa | 0.809 ± 0.008 Aa | 1.335 ± 0.232 Ab | 0.504 ± 0.024 Ca | 1.170 ± 0.071 Ab |
| Enterobacter sp. (BA110R) | 0.801 ± 0.001 Aa | 1.500 ± 0.080 Ba | 0.536 ± 0.011 Cb | 2.370 ± 0.152 Aa | 0.692 ± 0.029 Cb | 0.272 ± 0.158 Cb | 1.069 ± 0.162 Aa | 0.280 ± 0.264 Cb |
| Bacillus sp. (BA122R) | 0.778 ± 0.012 Ba | 0.962 ± 0.245 Da | 0.637 ± 0.048 Bb | 1.900 ± 0.073 Aa | 0.738 ± 0.007 Ba | 0.354 ± 0.074 Cb | 0.829 ± 0.035 Ba | 0.790 ± 0.024 Bb |
| Enterobacter asburiae (BA123R) | 0.802 ± 0.001 Aa | 1.579 ± 0.142 Ba | 0.536 ± 0.022 Ca | 1.830 ± 0.026 Aa | 0.782 ± 0.007 Aa | 0.993 ± 0.114 Bb | 0.618 ± 0.047 Ba | 1.190 ± 0.012 Ab |
| Enterobacter asburiae (BA203R) | 0.800 ± 0.004 Aa | 1.645 ± 0.049 Ba | 0.559 ± 0.016 Ca | 1.700 ± 0.027 Aa | 0.764 ± 0.011 Ba | 0.807 ± 0.124 Bb | 0.706 ± 0.063 Ba | 0.820 ± 0.019 Bb |
| Control | 0.781 ± 0.009 Ba | 1.156 ± 0.052 Ca | 0.630 ± 0.052 Bb | 1.430 ± 0.174 Aa | 0.802 ± 0.005 Aa | 0.130 ± 0.013 Cb | 0.689 ± 0.079 Ba | 0.040 ± 0.172 Db |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitorino, L.C.; Silva, F.O.d.; Cruvinel, B.G.; Bessa, L.A.; Rosa, M.; Souchie, E.L.; Silva, F.G. Biocontrol Potential of Sclerotinia sclerotiorum and Physiological Changes in Soybean in Response to Butia archeri Palm Rhizobacteria. Plants 2020, 9, 64. https://0-doi-org.brum.beds.ac.uk/10.3390/plants9010064
Vitorino LC, Silva FOd, Cruvinel BG, Bessa LA, Rosa M, Souchie EL, Silva FG. Biocontrol Potential of Sclerotinia sclerotiorum and Physiological Changes in Soybean in Response to Butia archeri Palm Rhizobacteria. Plants. 2020; 9(1):64. https://0-doi-org.brum.beds.ac.uk/10.3390/plants9010064
Chicago/Turabian StyleVitorino, Luciana Cristina, Fellipe Oliveira da Silva, Bárbara Gonçalves Cruvinel, Layara Alexandre Bessa, Márcio Rosa, Edson Luiz Souchie, and Fabiano Guimarães Silva. 2020. "Biocontrol Potential of Sclerotinia sclerotiorum and Physiological Changes in Soybean in Response to Butia archeri Palm Rhizobacteria" Plants 9, no. 1: 64. https://0-doi-org.brum.beds.ac.uk/10.3390/plants9010064