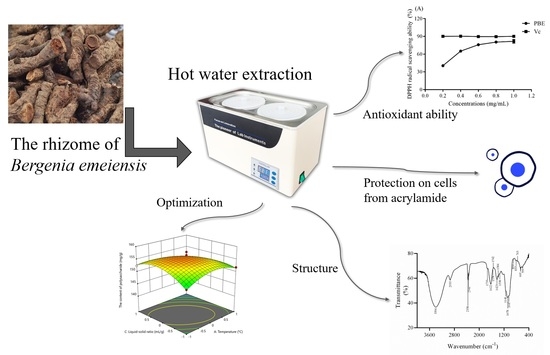
Optimized Extraction of Polysaccharides from Bergenia emeiensis Rhizome, Their Antioxidant Ability and Protection of Cells from Acrylamide-induced Cell Death
Abstract
:1. Introduction
2. Results
2.1. Single-factor Experiment Analysis
2.2. Analysis of the Response Surface
2.3. Characteristics and Chemical Composition of Polysaccharides
2.4. Antioxidant Ability in vitro
2.5. Protection Against Acrylamide
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Determination of Polysaccharides
4.3. Extraction Polysaccharides from Bergenia emeiensis
4.4. Response Surface Design
4.5. Purification of Polysaccharides
4.6. Characterization of Purified Polysaccharides
4.6.1. FT-IR Assay
4.6.2. Determination of Protein
4.6.3. Determination of Molecular Weight
4.7. Determination of Antioxidant Ability
4.7.1. Clearance Ability on DPPH Radicals
4.7.2. Clearance Ability on ABTS Radical
4.7.3. Fe2+ Chelating Ability
4.7.4. Total Reducing Power
4.8. The Protection Against Acrylamide
4.8.1. The Cell Culture
4.8.2. The Toxicity of PBE on Cells
4.8.3. The Remission on Acrylamide-induced Damage
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Joshi, V.S.; Parekh, B.B.; Joshi, M.J.; Vaidya, A.D.B. Inhibition of the growth of urinary calcium hydrogen phosphate dihydrate crystals with aqueous extracts of Tribulus terrestris and Bergenia ligulata. Urol. Res. 2005, 33, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Arshad, M.; Ahmad, M.; Saeed, M.; Ishaque, M. Ethnopharmacological survey of some medicinally important plants of Galliyat areas of NWFP, Pakistan. Asian J. Plant Sci. 2004, 3, 410–415. [Google Scholar] [CrossRef]
- Sinha, S.; Murugesan, T.; Maiti, K.; Gayen, J.R.; Pal, B.; Pal, M.; Saha, B.P. Antibacterial activity of Bergenia ciliate rhizomes. Fitoterpia 2001, 72, 550–552. [Google Scholar] [CrossRef]
- Uniyal, S.K.; Singh, K.N.; Jamwal, P.; Lal, B. Traditional use of medicinal plants among the tribal communities of Chhota Bhangal, Western Himalaya. J. Ethnobiol. Ethnomed. 2006, 2, 14–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Yoshida, T.; Hatano, T.; Fukushima, M.; Okuda, T. Galloylarbutin and other polyphenols from Bergenia purpurascens. Phytochemistry 1987, 26, 515–517. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, J.; Zhang, X.; Liu, Z.; Tsering, T.; Zhong, Y.; Nan, P. Chemical composition of the volatiles of three wild Bergenia species from western China. Flavour Frag. J. 2006, 21, 431–434. [Google Scholar] [CrossRef]
- Bohm, B.A.; Donevan, L.S.; Bhat, U.G. Flavonoids of some species of Bergenia, francoa, parnassia and lepuropetalon. Biochem. Syst. Ecol. 1986, 14, 75–77. [Google Scholar] [CrossRef]
- Yuldashev, M.P.; Batirov, È.K.; Malikov, V.M. Anthraquinones of Bergenia hissarica. Chem. Nat. Compd. 1993, 29, 543–544. [Google Scholar] [CrossRef]
- Mohib, U.K.; Muhammad, N.; Muhammad, S.; Shicong, Z.; Madiha, R.; Sundas, F.; Robina, M.; Yulin, D.; Rongji, D. A review on structure, extraction, and biological activities of polysaccharides isolated from Cyclocarya paliurus (Batalin) Iljinskaja. Int. J. Biol. Macromol. 2020, 156, 420–429. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G. Antitumor activity of polysaccharides: An Overview. Curr. Drug Targets 2018, 19, 89–96. [Google Scholar] [CrossRef]
- Wu, G.H.; Hu, T.; Li, Z.Y.; Huang, Z.L.; Jiang, J.G. In vitro antioxidant activities of the polysaccharides from Pleurotus tuber-regium (fr.) sing. Food Chem. 2014, 148, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhu, M.; Geng, X.; Wang, H.; Tb, T.B. Characterization of polysaccharides with antioxidant and hepatoprotective activities from the edible mushroom oudemansiella radicata. Molecules 2017, 22, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deniz, B.; Ismail, H.B. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–846. [Google Scholar] [CrossRef]
- Said, K.A.M.; Amin, M.A.M. Overview on the response surface methodology (RSM) in extraction processes. J. Appl. Sci. Process. Eng. 2015, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.J.; Wei, X.; Xu, J.; Chen, B.J.; Zhao, D.Y.; Cui, S.; Zhou, T. Carboxymethylated degraded polysaccharides from Enteromorpha prolifera: Preparation and in vitro antioxidant activity. Food Chem. 2017, 215, 76–83. [Google Scholar] [CrossRef]
- Guo, Q.; Cui, S.W.; Kang, J.; Ding, H.; Wang, Q.; Wang, C. Non-starch polysaccharides from American ginseng: Physicochemical investigation and structural characterization. Food Hydrocolloid. 2015, 44, 320–327. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, Y. Purification, chemical characterization and antioxidant activities of a novel polysaccharide from Auricularia polytricha. Int. J. Biol. Macromol. 2018, 120, 1087–1092. [Google Scholar] [CrossRef]
- Su, Y.; Li, L. Structural Characterization and antioxidant activity of polysaccharide from four Auriculariales. Carbohyd. Polym. 2019, 229, 115407. [Google Scholar] [CrossRef]
- Lin, Y.; Zeng, H.; Wang, K.; Lin, H.; Li, P.; Huang, Y.; Zhou, S.; Zhang, W.; Chen, T.; Fan, H. Microwave-assisted aqueous two-phase extraction of diverse polysaccharides from Lentinus edodes: Process optimization, structure characterization and antioxidant activity. Int. J. Biol. Macromol. 2019, 136, 305–315. [Google Scholar] [CrossRef]
- Kardošová, A.; Machová, E. Antioxidant activity of medicinal plant polysaccharides. Fitoterapia 2006, 77, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, Y.; Li, D.; Liu, C.; Jin, M.; Bian, J.; Lv, M.; Sun, Y.; Zhang, L.; Gao, P. The neuroprotective and antioxidant profiles of selenium containing polysaccharides from the fruit of Rosa laevigata. Food Funct. 2018, 9, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, Y.; Duan, X.; Tang, T.; Shen, Y.; Hu, B.; Liu, A.; Chen, H.; Li, C.; Liu, Y. Characterization and antioxidant activities of polysaccharides from thirteen boletus mushrooms. Int. J. Biol. Macromol. 2018, 113, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Liu, J.; Chen, X.; Zhang, J.; Wang, J. Purification, structure and anti-oxidation of polysaccharides from the fruit of Nitraria tangutorum Bobr. RSC Adv. 2018, 8, 11731–11743. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhou, Y.; Liu, M.; Wang, Q.; Li, Y. Extraction optimization, characterization, antioxidant and immunomodulatory activities of a novel polysaccharide from the wild mushroom Paxillus involutus. Int. J. Biol. Macromol. 2018, 112, 326–332. [Google Scholar] [CrossRef]
- Wang, D.; Fan, B.; Wang, Y.; Zhang, L.; Wang, F. Optimum extraction, characterization, and antioxidant activities of polysaccharides from flowers of Dendrobium devonianum. Int. J. Anal. Chem. 2018, 2018, 3013497. [Google Scholar] [CrossRef] [Green Version]
- Majeed, M.; Hussain, A.I.; Chatha, S.A.S.; Khosa, M.K.K.; Kamal, G.M.; Kamal, M.A.; Zhang, X.; Liu, M. Optimization protocol for the extraction of antioxidant components from Origanum vulgare leaves using response surface methodology. Saudi J. Biol. Sci. 2016, 23, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Sauer, H.; Wartenberg, M.; Hescheler, J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell. Physiol. Biochem. 2001, 11, 173–186. [Google Scholar] [CrossRef]
- Vincent, A.M.; Russell, J.W.; Low, P.; Feldman, E.L. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr. Rev. 2004, 25, 612–628. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, B.; Tang, C.; Gou, Y.; Chen, H.; Wang, Y.; Jin, C.; Liu, J.; Niu, F.; Kan, J.; et al. Characterization, antioxidant activity and hepatoprotective effect of purple sweetpotato polysaccharides. Int. J. Biol. Macromol. 2018, 115, 69–76. [Google Scholar] [CrossRef]
- Shang, H.; Wang, M.; Li, R.; Duan, M.; Wu, H.; Zhou, H. Extraction condition optimization and effects of drying methods on physicochemical properties and antioxidant activities of polysaccharides from Astragalus Cicer L. Sci. Rep. 2018, 8, 3359. [Google Scholar] [CrossRef]
- Rjeibi, I.; Feriani, A.; Hentati, F.; Hfaiedh, N.; Michaud, P.; Pierre, G. Structural characterization of water-soluble polysaccharides from Nitraria retusa fruits and their antioxidant and hypolipidemic activities. Int. J. Biol. Macromol. 2019, 129, 422–432. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid. Med. Cell Longev. 2016, 2016, e5692852. [Google Scholar] [CrossRef] [Green Version]
- Yousef, M.I.; El-Demerdash, F.M. Acrylamide-induced oxidative stress and biochemical perturbations in rats. Toxicology 2006, 219, 133–141. [Google Scholar] [CrossRef]
- Barber, D.S.; Hunt, J.R.; Ehrich, M.F.; Lehning, E.J.; LoPachin, R.M. Metabolism, toxicokinetics and hemoglobin adduct formation in rats following subacute and subchronic acrylamide dosing. NeuroToxicology 2001, 22, 341–353. [Google Scholar] [CrossRef]
- Alturfan, A.A.; Tozan-Beceren, A.; Şehirli, A.Ö.; Demiralp, E.; Şener, G.; Omurtag, G.Z. Resveratrol ameliorates oxidative DNA damage and protects against acrylamide-induced oxidative stress in rats. Mol. Biol. Rep. 2011, 39, 4589–4596. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Liu, Y.; Jia, L.; Jiang, L.P.; Geng, C.Y.; Yao, X.F.; Kong, Y.; Jiang, B.N.; Zhong, L.F. Curcumin attenuates acrylamide-induced cytotoxicity and genotoxicity in HepG2 cells by ROS scavenging. J. Agr. Food Chem. 2008, 56, 12059–12063. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 163, 76–85. [Google Scholar] [CrossRef]
- Brand, W.W.M.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Xu, G.Y.; Liao, A.M.; Huang, J.H.; Zhang, J.G.; Thakur, K.; Wei, Z.J. Evaluation of structural, functional, and anti-oxidant potential of differentially extracted polysaccharides from potatoes peels. Int. J. Biol. Macromol. 2019, 129, 778–785. [Google Scholar] [CrossRef]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]






| Run | A-Temperature (°C) | B-Time (min) | C-Liquid-Solid Ratio (mL/g) | Y-Yield of Polysaccharides (mg/g) |
|---|---|---|---|---|
| 1 | 40(0) | 30(−1) | 10(−1) | 154.03 |
| 2 | 40(0) | 90(1) | 30(1) | 146.55 |
| 3 | 40(0) | 90(1) | 10(−1) | 151.91 |
| 4 | 40(0) | 60(0) | 20(0) | 157.90 |
| 5 | 30(−1) | 60(0) | 10(−1) | 150.05 |
| 6 | 30(−1) | 60(0) | 30(1) | 152.44 |
| 7 | 40(0) | 30(−1) | 30(1) | 156.89 |
| 8 | 50(1) | 60(0) | 10(−1) | 151.82 |
| 9 | 50(1) | 30(−1) | 20(0) | 157.98 |
| 10 | 30(−1) | 30(−1) | 20(0) | 154.24 |
| 11 | 40(0) | 60(0) | 20(0) | 155.55 |
| 12 | 40(0) | 60(0) | 20(0) | 155.01 |
| 13 | 30(−1) | 90(1) | 20(0) | 154.58 |
| 14 | 40(0) | 60(0) | 20(0) | 155.20 |
| 15 | 50(1) | 90(1) | 20(0) | 144.39 |
| 16 | 50(1) | 60(0) | 30(1) | 144.95 |
| 17 | 40(0) | 60(0) | 20(0) | 156.65 |
| Experim. | 43.11 | 30 | 20.69 | 159.25 |
| Actual | 43 | 30 | 21 | 158.34 ± 0.98 |
| Source | Sum of Squares | Mean Square | F-Value | p-Value |
|---|---|---|---|---|
| Model | 283.37 | 31.49 | 34.15 | <0.0001 *** |
| A | 18.56 | 18.56 | 20.13 | 0.0028 ** |
| B | 82.57 | 82.57 | 89.55 | <0.0001 *** |
| C | 6.12 | 6.12 | 6.63 | 0.0367 * |
| AB | 48.48 | 48.48 | 52.57 | 0.0002 ** |
| AC | 21.47 | 21.47 | 23.28 | 0.0019 ** |
| BC | 16.87 | 16.87 | 18.3 | 0.0037 ** |
| A2 | 35.34 | 35.34 | 38.32 | 0.0004 ** |
| B2 | 0.5713 | 0.5713 | 0.6196 | 0.457 |
| C2 | 47.26 | 47.26 | 51.25 | 0.0002 ** |
| Residual | 6.45 | 0.9221 | ||
| Lack of Fit | 0.6367 | 0.2122 | 0.1459 | 0.9271 |
| R2 | 0.9777 | |||
| Adj R2 | 0.9491 | |||
| C.V. % | 0.6278 |
| Components | Content |
|---|---|
| Polysaccharides | 95.97 ± 0.92% |
| Protein | 0.83 ± 0.025% |
| Molecular weight | 1.42 × 105 Da |
| Factors | Coding Level | ||
|---|---|---|---|
| Extraction time | −1(30 min) | 0(60 min) | 1(90 min) |
| Extraction temperature | −1(30 °C) | 0(40 °C) | 1(50 °C) |
| Liquid-solid ratio | −1(10 mL/g) | 0(20 mL/g) | 1(30 mL/g) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, C.; Feng, S. Optimized Extraction of Polysaccharides from Bergenia emeiensis Rhizome, Their Antioxidant Ability and Protection of Cells from Acrylamide-induced Cell Death. Plants 2020, 9, 976. https://0-doi-org.brum.beds.ac.uk/10.3390/plants9080976
Zeng C, Feng S. Optimized Extraction of Polysaccharides from Bergenia emeiensis Rhizome, Their Antioxidant Ability and Protection of Cells from Acrylamide-induced Cell Death. Plants. 2020; 9(8):976. https://0-doi-org.brum.beds.ac.uk/10.3390/plants9080976
Chicago/Turabian StyleZeng, Chen, and Shiling Feng. 2020. "Optimized Extraction of Polysaccharides from Bergenia emeiensis Rhizome, Their Antioxidant Ability and Protection of Cells from Acrylamide-induced Cell Death" Plants 9, no. 8: 976. https://0-doi-org.brum.beds.ac.uk/10.3390/plants9080976