Recent Advances of the Polymer Micro/Nanofiber Fluorescence Waveguide
Abstract
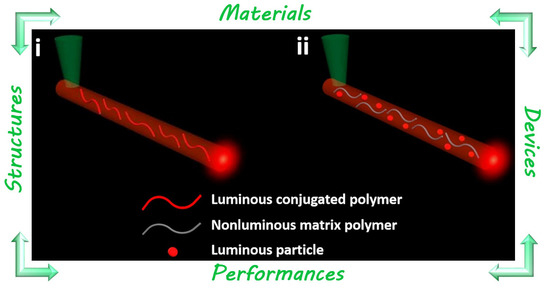
:1. Introduction to Polymer Optical Micro/Nanofibers
2. Polymer Micro/Nanofiber Fluorescence Waveguides
2.1. Fabrication of Polymer Optical Micro/Nanofibers
2.2. Low-Transmission Loss of Polymer Micro/Nanofiber Waveguides
2.3. Modulation of Polymer Micro/Nanofiber Waveguides
2.4. Application of the Polymer Micro/Nanofiber Fluorescence Waveguide
3. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miller, S.E. Integrated Optics: An Introduction. Bell Labs Tech. J. 1969, 48, 2059–2069. [Google Scholar] [CrossRef]
- Tien, P.K. Light Waves in Thin Films and Integrated Optics. Appl. Opt. 1971, 10, 2395–2413. [Google Scholar] [CrossRef] [PubMed]
- Barrelet, C.J.; Greytak, A.B.; Lieber, C.M. Nanowire Photonic Circuit Elements. Nano Lett. 2004, 4, 1981–1985. [Google Scholar] [CrossRef]
- Sanders, A.W.; Routenberg, D.A.; Wiley, B.J.; Xia, Y.N.; Dufresne, E.R.; Reed, M.A. Observation of Plasmon Propagation, Redirection, and Fan-Out in Silver Nanowires. Nano Lett. 2006, 6, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Gargas, D.; Yang, P. Nanowire photonics. Nat. Photonics 2009, 3, 569–576. [Google Scholar] [CrossRef]
- Kirchain, R.; Kimerling, L. A roadmap for nanophotonics. Nat. Photonics 2007, 1, 303–305. [Google Scholar] [CrossRef]
- Tong, L.; Gattass, R.; Ashcom, J.B.; He, S.; Lou, J.; Shen, M.; Maxwell, I.; Mazur, E. Subwavelength-diameter silica wires for low-loss optical wave guiding. Nature 2003, 426, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, H.J.; Dolev, S. Why future supercomputing requires optics. Nat. Photonics 2010, 4, 261–263. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Tong, L. Functionalized polymer nanofibers: A versatile platform for manipulating light at the nanoscale. Light-Sci. Appl. 2013, 2, e102. [Google Scholar] [CrossRef]
- Zhuang, X.; Ning, C.Z.; Pan, A. Composition and Bandgap-Graded Semiconductor Alloy Nanowires. Adv. Mater. 2012, 24, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, Q.; Fan, F.; Xu, H.; Wang, Z.L. Light Propagation in Curved Silver Nanowire Plasmonic Waveguides. Nano Lett. 2011, 11, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Heinze, S.; Jaeger, H.; Lopes, W. Top five in physics. Nature 2006, 441, 265. [Google Scholar]
- Brambilla, G.; Koizumi, F.; Feng, X.; Richardson, D.J. Compound-glass optical nanowires. Electron. Lett. 2005, 41, 400–402. [Google Scholar] [CrossRef]
- Tong, L.; Hu, L.; Zhang, J.; Qiu, J.; Yang, Q.; Lou, J.; Shen, Y.; He, J.; Ye, Z. Photonic nanowires directly drawn from bulk glasses. Opt. Express 2006, 14, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Radovanovic, P.V.; Barrelet, C.J.; Gradečak, S.; Qian, F.; Lieber, C.M. General synthesis of manganese-doped II–VI and III–V semiconductor nanowires. Nano Lett. 2005, 5, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Kuykendall, T.; Ulrich, P.; Aloni, S.; Yang, P. Complete composition tunability of InGaN nanowires using a combinatorial approach. Nat. Mater. 2007, 6, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, P. Direct observation of vapor–liquid–solid nanowire growth. J. Am. Chem. Soc. 2001, 123, 3165–3166. [Google Scholar] [CrossRef]
- Yoon, J.; Jung, Y.; Kim, J. A Combinatorial Approach for Colorimetric Differentiation of Organic Solvents Based on Conjugated Polymer-Embedded Electrospun Fibers. Adv. Funct. Mater. 2009, 19, 209–214. [Google Scholar] [CrossRef]
- Wang, H.; Liao, Q.; Fu, H.B.; Zeng, Y.; Jiang, Z.W.; Ma, J.S.; Yao, J.N. Ir(ppy)3 phosphorescent microrods and nanowires: Promising micro-phosphors. J. Mater. Chem. 2009, 19, 89–96. [Google Scholar] [CrossRef]
- Moran-Mirabal, J.M.; Slinker, J.D.; DeFranco, J.A.; Verbridge, S.S.; Llic, R.; Flores-Torres, S.; Abruña, H.; Malliaras, G.G.; Craighead, H.G. Electrospun Light-Emitting Nanofibers. Nat. Photonics 2007, 7, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Q.; Edel, J.B.; Bellan, L.M.; Craighead, H.G. Electrospun Polymer Nanofibers as Subwavelength Optical Waveguides Incorporating Quantum Dots. Small 2006, 2, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Jen, A.K.Y.; Dalton, L.R. Polymer-Based Optical Waveguides: Materials, Processing, and Devices. Adv. Mater. 2002, 14, 1339–1365. [Google Scholar] [CrossRef]
- O’Carroll, D.; Lieberwirth, I.; Redmond, G. Microcavity effects and optically pumped lasing in single conjugated polymer nanowires. Nat. Nanotechnol. 2007, 2, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Camposeo, A.; Persano, L.; Pisignano, D. Light-Emitting Electrospun Nanofibers for Nanophotonics and Optoelectronics. Macromol. Mater. Eng. 2013, 298, 487–503. [Google Scholar] [CrossRef]
- Vohra, V.; Giovanella, U.; Tubino, R.; Murata, H.; Botta, C. Electroluminescence from Conjugated Polymer Electrospun Nanofibers in Solution Processable Organic Light-Emitting Diodes. ACS Nano 2011, 5, 5572–5578. [Google Scholar] [CrossRef] [PubMed]
- Taboada, J.M.; An, D.; Shi, Z.; Maki, J.J.; Tang, S. Fabrication and testing of polyimide thermo-optic switches. Proc. SPIE 2000, 3952, 242–247. [Google Scholar]
- Kaino, T. Plastic optical fibers for near-infrared transmission. Appl. Phys. Lett. 1986, 48, 757–758. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, H.J.; Choi, J.H.; Koh, W.G.; Myoung, J.M.; Hur, J.H.; Park, J.J.; Cho, J.H.; Jeong, U. Periodic Array of Polyelectrolyte-Gated Organic Transistors from Electrospun Poly(3-hexylthiophene) Nanofibers. Nano Lett. 2010, 10, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Persano, L.; Camposeo, A.; Carro, P.D.; Fasano, V.; Moffa, M.; Manco, R.; D’Agostino, S.; Pisignano, D. Distributed Feedback Imprinted Electrospun Fiber Lasers. Adv. Mater. 2014, 26, 6542–6547. [Google Scholar] [CrossRef] [PubMed]
- Iacopino, D.; Redmond, G. Reversible modulation of photoluminescence from conjugated polymer nanotubes by incorporation of photochromic spirooxazine molecules. Chem. Commun. 2011, 47, 9170–9172. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, L.; Xia, Y.N.; Tong, L.M.; Xu, X.; Ying, Y.B. Polymer Nanofibers Embedded with Aligned Gold Nanorods: A New Platform for Plasmonic Studies and Optical Sensing. Nano Lett. 2012, 12, 3145–3150. [Google Scholar] [CrossRef] [PubMed]
- O’Carroll, D.; Iacopino, D.; O’Riordan, A.; Lovera, P.; O’Connor, É.; O’Brien, G.A.; Redmond, G. Poly(9,9-dioctylfluorene) Nanowires with Pronounced b-Phase Morphology: Synthesis, Characterization, and Optical Properties. Adv. Mater. 2008, 20, 42–48. [Google Scholar] [CrossRef]
- Wang, P.; Li, Z.Y.; Zhang, L.; Tong, L.M. Electron-beam-activated light-emitting polymer nanofibers. Opt. Lett. 2013, 38, 1040–1042. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Kappl, M.; Liebewirth, I.; Müller, M.; Kirchhoff, K.; Pisula, W.; Müllen, K. Organic Field-Effect Transistors based on Highly Ordered Single Polymer Fibers. Adv. Mater. 2012, 24, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, G.A.; Quinn, A.J.; Tanner, D.A.; Redmond, G. A Single Polymer Nanowire Photodetector. Adv. Mater. 2006, 18, 2379–2383. [Google Scholar] [CrossRef]
- Gu, F.X.; Zhang, L.; Yin, X.F.; Tong, L.M. Polymer Single-Nanowire Optical Sensors. Nano Lett. 2008, 8, 2757–2761. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Xia, H.Y.; Cheng, J.J.; Zou, G. Bioinspired Polarization Sensitive Photodetector System Using Orientation-Tunable PDA Microfbers Arrays. Macromol. Mater. Eng. 2016, 301, 1301–1306. [Google Scholar] [CrossRef]
- Morello, G.; Manco, R.; Moffa, M.; Persano, L.; Camposeo, A.; Pisignano, D. Multifunctional Polymer Nanofibers: UV Emission, Optical Gain, Anisotropic Wetting, and High Hydrophobicity for Next Flexible Excitation Sources. ACS Appl. Mater. Interfaces 2015, 7, 21907–21912. [Google Scholar] [CrossRef] [PubMed]
- O’Carroll, D.; Lieberwirth, I.; Redmond, G. Melt-Processed Polyfluorene Nanowires as Active Waveguides. Small 2007, 3, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.X.; Yu, H.K.; Wang, P.; Yang, Z.Y.; Tong, L.M. Light-Emitting Polymer Single Nanofibers via Waveguiding Excitation. ACS Nano 2010, 4, 5332–5338. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Xiao, Y.; Wang, P.; Zhang, L.; Liu, Y.X.; Tong, L.M. Quantum-Dot-Doped Polymer Nanofibers for Optical Sensing. Adv. Mater. 2011, 23, 3770–3774. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.L.; Chen, Y.K.; Jiang, H.; Li, J.G.; Zou, G.; Zhang, Q.J.; Zhang, D.G.; Wang, P.; Ming, H. Optical Waveguide Based on a Polarized Polydiacetylene Microtube. Adv. Mater. 2014, 36, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y.N. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Fasano, V.; Polini, A.; Morello, G.; Moffa, M.; Camposeo, A.; Pisignano, D. Bright Light Emission and Waveguiding in Conjugated Polymer Nanofibers Electrospun from Organic Salt Added Solutions. Macromolecules 2013, 46, 5935–5942. [Google Scholar] [CrossRef] [PubMed]
- Fasano, V.; Moffa, M.; Camposeo, A.; Persano, L.; Pisignano, D. Controlled Atmosphere Electrospinning of Organic Nanofibers with Improved Light Emission and Waveguiding Properties. Macromolecules 2015, 48, 7803–7809. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Q.; Li, B.J. Wavelength-converted wave-guiding in dye-doped polymer nanofibers. Sci. Rep. 2013, 3, 1674. [Google Scholar] [CrossRef] [PubMed]
- Camposeo, A.; Benedetto, F.D.; Stabile, R.; Neves, A.A.R.; Cingolani, R.; Pisignano, D. Laser Emission from Electrospun Polymer Nanofibers. Small 2009, 5, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.X.; Xia, H.Y.; Zhang, D.G.; Chen, J.X.; Zhu, L.F.; Wang, Y.; Yang, E.C.; Zang, T.Y.; Wen, X.L.; Zou, G.; et al. Bloch surface waves confined in one dimension with a single polymeric nanofiber. Nat. Commun. 2017, 8, 14330. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, Y.F.; Liu, Y.Y.; Zou, G.; Gao, Z.; Wang, F. Cooperative Supramolecular Polymerization of Fluorescent Platinum Acetylides for Optical Waveguide Applications. Angew. Chem. Int. Ed. 2017, 56, 12466–12470. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, F.D.; Mele, E.; Camposeo, A.; Athanassiou, A.; Cingolani, R.; Pisignano, D. Photoswitchable Organic Nanofibers. Adv. Mater. 2008, 20, 314–318. [Google Scholar] [CrossRef]
- Zhu, M.Q.; Zhu, L.Y.; Han, J.J.; Wu, W.W.; Hurst, J.K.; Li, A.D.Q. Spiropyran-Based Photochromic Polymer Nanoparticles with Optically Switchable Luminescence. J. Am. Chem. Soc. 2006, 128, 4303–4309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.L.; Li, L.; Sun, X.M.; Liu, Y.P.; Luo, B.; Wang, C.C.; Bao, Y.P.; Xu, H.; Peng, H.S. Magnetochromatic Polydiacetylene by Incorporation of Fe3O4 Nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 5486–5489. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.M.; Chen, T.; Huang, S.Q.; Li, L.; Peng, H.S. Chromatic polydiacetylene with novel sensitivity. Chem. Soc. Rev. 2010, 39, 4244–4257. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.Y.; Chen, Y.K.; Yang, G.; Zou, G.; Zhang, Q.J.; Zhang, D.G.; Wang, P.; Ming, H. Optical Modulation of Waveguiding in Spiropyran-Functionalized Polydiacetylene Microtube. ACS Appl. Mater. Interfaces 2014, 6, 15466–15471. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, Y.; Xia, H.Y.; Zou, G.; Zhang, Q.J. Multiconfigurable logic gate operation in 1D polydiacetylene microtube waveguide. RSC Adv. 2016, 6, 53794–53799. [Google Scholar] [CrossRef]
- Xia, H.Y.; Wang, R.X.; Liu, Y.Y.; Cheng, J.J.; Zou, G.; Zhang, Q.J.; Zhang, D.G.; Wang, P.; Ming, H.; Badugu, R.; et al. Active Polymer Microfiber with Controlled Polarization Sensitivity. Adv. Opt. Mater. 2016, 4, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jiang, X.S.; Gu, F.X.; Ma, Z.; Zhang, J.Y.; Tong, L.M. Polymer Micro or Nanofibers for Optical Device Applications. J. Appl. Polym. Sci. 2008, 110, 1080–1084. [Google Scholar] [CrossRef]
- Guo, X.; Ying, Y.B.; Tong, L.M. Photonic Nanowires: From Subwavelength Waveguides to Optical Sensors. Acc. Chem. Res. 2014, 47, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yu, J.; Jiang, S.; Zhang, C.; Sun, Q.; Wang, M.; Zhou, H.; Li, C.; Man, B.; Lei, F. High stability luminophores: Fluorescent CsPbX3 (X=Cl, Br and I) nanofiber prepared by one-step electrospinning method. Opt. Express 2018, 26, 20649–20660. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.B.; Wang, W. Human Placental MicroRNAs and Preeclampsia. Biol. Reprod. 2013, 88, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Kim, J.H.; Jung, J.; Chung, B.H. Nuclease-resistant DNA aptamer on gold nanoparticles for the simultaneous detection of Pb2þ and Hg2þ in human serum. Biosens. Bioelectron. 2013, 41, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Qiu, D.; Yang, G.; Wang, M.Q.; Zhang, Q.J.; Wang, P.; Ming, H.; Zhang, D.G.; Yu, Y.; Zou, G.; et al. Selective and sensitive detection of MiRNA-21 based on gold-nanorod functionalized polydiacetylene microtube waveguide. Biosens. Bioelectron. 2016, 85, 198–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.Q.; Li, B.H.; Li, W.W.; Zhao, J.; Sun, S.G.; Pang, Y. ICT-not-quenching near infrared ratiometric fluorescent detection of picric acid in aqueous media. Chem. Commun. 2013, 49, 4764–4766. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Peng, H.N.; Liu, T.H.; Yang, M.N.; Zhang, Y.; Fang, Y. A novel picric acid film sensor via combination of the surface enrichment effect of chitosan films and the aggregation-induced emission effect of siloles. J. Mater. Chem. 2009, 19, 7347–7353. [Google Scholar] [CrossRef]
- Yang, G.; Hu, W.L.; Xia, H.Y.; Zou, G.; Zhang, Q.J. Highly selective and reproducible detection of picric acid in aqueous media, based on a polydiacetylene microtube optical waveguide. J. Mater. Chem. A 2014, 2, 15560–15565. [Google Scholar] [CrossRef]
- Kemp, N.T.; McGrouther, D.; Cochrane, J.W.; Newbury, R. Bridging the Gap: Polymer Nanowire Devices. Adv. Mater. 2007, 19, 2634–2638. [Google Scholar] [CrossRef]



















© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, H.; Chen, T.; Hu, C.; Xie, K. Recent Advances of the Polymer Micro/Nanofiber Fluorescence Waveguide. Polymers 2018, 10, 1086. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10101086
Xia H, Chen T, Hu C, Xie K. Recent Advances of the Polymer Micro/Nanofiber Fluorescence Waveguide. Polymers. 2018; 10(10):1086. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10101086
Chicago/Turabian StyleXia, Hongyan, Tingkuo Chen, Chang Hu, and Kang Xie. 2018. "Recent Advances of the Polymer Micro/Nanofiber Fluorescence Waveguide" Polymers 10, no. 10: 1086. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10101086