Facile Route for Bio-Phenol Siloxane Synthesis via Heterogeneous Catalytic Method and its Autonomic Antibacterial Property
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
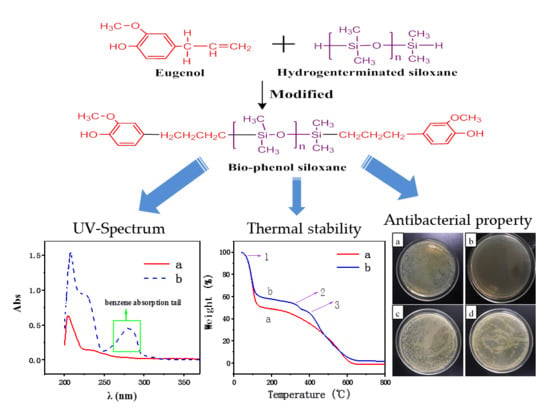
2.2. Synthesis of Bio-Phenol Siloxane
2.3. Nuclear Magnetic Resonance Hydrogen (1H NMR) Spectrometer Analysis
2.4. Fourier Transform Infrared (FT–IR) Analysis
2.5. Ultraviolet–Visible (UV) Analysis
2.6. Thermal Analysis
2.7. Antimicrobial Activity
3. Results and Discussions
3.1. Analysis of Catalytic Activity
3.1.1. Catalytic Activity of Pt-Al2O3
3.1.2. Catalytic Activity of Pt-SiO2
3.1.3. Catalytic Activity of Pt-CNT
3.2. FT–IR Analysis
3.3. UV Analysis
3.4. Thermal Analysis
3.5. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karapinar, M.; Şahika, E.A. Inhibition of foodborne pathogens by thymol, eugenol, menthol and anethole. Int. J. Food Microbiol. 1987, 4, 161–166. [Google Scholar] [CrossRef]
- Shao, Y.; Wu, C.; Wu, T.; Li, Y.; Chen, S.; Yuan, C.; Hu, Y. Eugenol-chitosan nanoemulsions by ultrasound-mediated emulsification: Formulation, characterization and antimicrobial activity. Carbohydr. Polym. 2018, 193, 144. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.S.; Feng, Z.; Ji, B.P.; Pei, R.S.; Zhou, F.; Ji, B.P.; Xu, J. Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. J. Food. Sci. 2010, 74, M379–M383. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Murakami, K.; Yoshino, M. Antioxidant action of eugenol compounds: Role of metal ion in the inhibition of lipid peroxidation. Food Chem. Toxicol. 2005, 43, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Thirukumaran, P.; Parveen, A.S.; Sarojadevi, M. Synthesis of eugenol-based polybenzoxazine-POSS nanocomposites for low dielectric applications. Polym. Compos. 2015, 36, 1973–1982. [Google Scholar] [CrossRef]
- Roland, K.; Robert, M.; Michael, F.; Thorsten, S. Use of Eugenol Polyethers and Eugenol Polyethers Siloxanes as Wetting Agents. U.S. Patent 9993766B2, June 2018. [Google Scholar]
- Cheng, C.; Li, Y.; Zhang, X.; Li, J. Eugenol-based non-isocyanate polyurethane and polythiourethane. Iran. Polym. J. 2017, 26, 1–11. [Google Scholar] [CrossRef]
- Fujisawa, S.; Atsumi, T.; Kadoma, Y.; Sakagami, H. Antioxidant and prooxidant action of eugenol-related compounds and their cytotoxicity. Toxicology 2002, 177, 39–54. [Google Scholar] [CrossRef]
- Dai, J.Y.; Yang, S.M.; Teng, N.; Liu, Y.; Liu, X.Q.; Zhu, J.; Zhao, J. Synthesis of Eugenol-Based Silicon-Containing Benzoxazines and Their Applications as Bio-Based Organic Coatings. Coatings 2018, 8, 88. [Google Scholar] [CrossRef]
- Miao, J.T.; Yuan, L.; Guan, Q.; Liang, G.; Gu, A. Biobased epoxy resin derived from eugenol with excellent integrated performances and high renewable carbon content. Polym. Int. 2018, 67, 1194–1202. [Google Scholar] [CrossRef]
- Mangeon, C.; Modjinou, T.; Rios de Anda, A.; Thevenieau, F.; Renard, E.; Langlois, V. Renewable semi-interpenetrating polymer networks based on vegetable oils used as plasticized systems of poly(3-hydroxyalkanoate)s. ACS Sustain. Chem. Eng. 2018, 6, 5034–5042. [Google Scholar] [CrossRef]
- da Silva, F.F.M.; Monte, F.J.Q.; de Lemos, T.L.G.; do Nascimento, P.G.G.; de Medeiros Costa, A.K.; de Paiva, L.M.M. Eugenol derivatives: Synthesis, characterization, and evaluation of antibacterial and antioxidant activities. Chem. Cent. J. 2018, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.N.; Stein, J.; Gao, Y.; Colborn, R.E.; Hutchins, G. Platinum catalysts used in the silicones industry. Platin. Met. Rev. 1997, 41, 66–75. [Google Scholar]
- Januszewski, R.; Kownacki, I.; Maciejewski, H.; Marciniec, B. An efficient catalytic and solvent-free method for the synthesis of mono-organofunctionalized 1,1,3,3-tetramethyldisiloxane derivatives. J. Organomet. Chem. 2017, 846, 263–268. [Google Scholar] [CrossRef]
- Xiao, J.J.; Qiu, Z.M.; He, W.J.; Du, C.C.; Zhou, W. Research progress of platinum-catalysts supported by inorganic support-supported hydrosilylation. Chin. J. Org. Chem. 2016, 36, 987–999. [Google Scholar] [CrossRef]
- Calvillo, L.; Gangeri, M.; Perathoner, S.; Centi, G.; Moliner, R.; Lázaro, M.J. Effect of the support properties on the preparation and performance of platinum catalysts supported on carbon nanofibers. J. Power. Sources 2009, 192, 144–150. [Google Scholar] [CrossRef]
- Cui, X.; Jun, G.K.; Dai, X.; Kreyenschulte, C.; Pohl, M.M.; Wohlrab, S.; Shi, F.; Brückner, A.; Beller, M. Synthesis of single atom based heterogeneous platinum catalysts: High selectivity and activity for hydrosilylation reactions. Acs. Cent. Sci. 2017, 3, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Gama-Lara, S.A.; Morales-Luckie, R.; Argueta-Figueroa, L.; Hinestroza, J.P.; García-Orozco, I.; Natividad, R. Synthesis, characterization, and catalytic activity of platinum nanoparticles on bovine-bone powder: A novel support. J. Nanomater. 2018. [Google Scholar] [CrossRef]
- Li, W.; Liang, C.; Zhou, W.; Qiu, J.; Zhou, Z.; Sun, G.; Xin, Q. Preparation and characterization of multiwalled carbon nanotube-supported platinum for cathode catalysts of direct methanol fuel cells. J. Phys. Chem. B. 2003, 107, 149–154. [Google Scholar] [CrossRef]
- Wu, N.; Li, B.; Liu, J.; Zuo, S.; Zhao, Y. Preparation and catalytic performance of a novel highly dispersed bifunctional catalyst Pt@Fe-MCM-41. RSC Adv. 2016, 6, 13461–13468. [Google Scholar] [CrossRef]
- Zgolicz, P.D.; Stassi, J.P.; Yañez, M.J.; Scelza, O.A.; de Miguel, S.R. Influence of the support and the preparation methods on the performance in citral hydrogenation of Pt-based catalysts supported on carbon nanotubes. J. Catal. 2012, 290, 37–54. [Google Scholar] [CrossRef]
- Yang, H.; Deng, J.; Liu, Y.; Xie, S.; Wu, Z.; Dai, H. Preparation and catalytic performance of Ag, Au, Pd or Pt nanoparticles supported on 3DOM CeO2-Al2O3 for toluene oxidation. J. Mol. Catal. A-Chem. 2016, 414, 9–18. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, H.; Wang, B.; Kong, Y.; Chen, J. Silver-incorporated mussel-inspired polydopamine coatings on mesoporous silica as an efficient nanocatalyst and antimicrobial agent. ACS Appl. Mater. Interfaces 2018, 10, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.H. Relationship between Antibacterial Activity and Chemical Structure of Cinnamaldehyde, Eugenol and its Structural Analogues. M.S. Thesis, Xiangtan University, 2013. [Google Scholar]








| Samples | Catalys (ppm) | Temperature (°C) | Appearance | Yield (%) |
|---|---|---|---|---|
| A-1 | 100 | 80 | Stratified, the bottom layer was unreactive eugenol | - |
| A-2 | 100 | 100 | Not stratified, as colorless turbid liquid | 36 |
| A-3 | 100 | 120 | Not stratified, as pale yellowish turbid liquid | - |
| A-4 | 100 | 140 | Not stratified, as yellowish turbid liquid | - |
| A-5 | 100 | 160 | Not stratified, as yellow turbid liquid | - |
| Samples | Catalyst (ppm) | Temperature (°C) | Appearance | Yield (%) |
|---|---|---|---|---|
| A-6 | 50 | 100 | Stratified, the bottom layer was unreactive eugenol | - |
| A-7 | 100 | 100 | Not stratified, as colorless turbid liquid | - |
| A-8 | 100 | 100 | Not stratified, as colorless turbid liquid | - |
| A-9 | 200 | 100 | Not stratified, as colorless turbid liquid | - |
| A-10 | 500 | 100 | Not stratified, as colorless turbid liquid | 76 |
| Samples | Catalyst (ppm) | Temperature (°C) | Appearance | Yield (%) |
|---|---|---|---|---|
| B-1 | 100 | 60 | Stratified, the bottom layer was unreactive eugenol | - |
| B-2 | 100 | 80 | Not stratified, as colorless turbid liquid | - |
| B-3 | 100 | 100 | Not stratified, as yellowish, little transparent liquid | 66 |
| Samples | Catalyst (ppm) | Temperature (°C) | Appearance | Yield (%) |
|---|---|---|---|---|
| B-4 | 10 | 80 | Not stratified, as colorless turbid liquid | - |
| B-5 | 50 | 80 | Not stratified, as colorless turbid liquid | - |
| B-6 | 100 | 80 | Not stratified, as colorless turbid liquid | - |
| B-7 | 200 | 80 | Not stratified, as colorless, slight transparent liquid | 80 |
| Samples | Catalyst (ppm) | Temperature (°C) | Appearance | Yield (%) |
|---|---|---|---|---|
| C-1 | 100 | 60 | Not stratified, as colorless and slight transparent liquid | - |
| C-2 | 100 | 80 | Not stratified, as colorless transparent liquid | 97 |
| C-3 | 100 | 100 | Not stratified, as yellowish and slight transparent liquid | - |
| Samples | Catalyst (ppm) | Temperature (°C) | Appearance | Yield (%) |
|---|---|---|---|---|
| C-4 | 30 | 80 | Not stratified, as colorless and slight transparent liquid | - |
| C-5 | 50 | 80 | Not stratified, as colorless and slight transparent liquid | - |
| C-6 | 100 | 80 | Not stratified, as colorless transparent liquid | 97 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, X.; Ge, X.; Ji, J.; Liang, W.; Chen, X.; Ge, J. Facile Route for Bio-Phenol Siloxane Synthesis via Heterogeneous Catalytic Method and its Autonomic Antibacterial Property. Polymers 2018, 10, 1151. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10101151
Pang X, Ge X, Ji J, Liang W, Chen X, Ge J. Facile Route for Bio-Phenol Siloxane Synthesis via Heterogeneous Catalytic Method and its Autonomic Antibacterial Property. Polymers. 2018; 10(10):1151. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10101151
Chicago/Turabian StylePang, Xiaoyan, Xin Ge, Jianye Ji, Weijie Liang, Xunjun Chen, and Jianfang Ge. 2018. "Facile Route for Bio-Phenol Siloxane Synthesis via Heterogeneous Catalytic Method and its Autonomic Antibacterial Property" Polymers 10, no. 10: 1151. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10101151