Soy Protein-Based Composite Hydrogels: Physico-Chemical Characterization and In Vitro Cytocompatibility
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
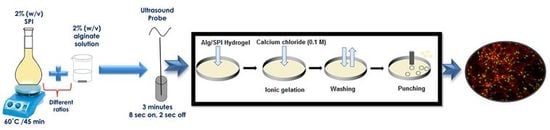
2.1.1. Sonochemical Preparation of Hydrogels
2.1.2. Preparation of Hydrogel Films
2.1.3. Physico-Chemical Characterization of Films
2.1.3.1. Water Uptake
2.1.3.2. Swelling and Degradation Behavior
2.1.4. Fourier Transform Infrared Spectroscopy
2.1.5. Scanning Electron Microscopy
2.1.6. Nano-Indentation
2.1.7. Effect of Incorporation of Bioactive Glass into Alg/SPI Hydrogel Films
2.1.8. Cell Response to Hydrogels
2.1.8.1. Cells
2.1.8.2. Cell Seeding onto 2D Hydrogels
2.1.8.3. Metabolic Activity of Cells
2.1.8.4. Cell Staining
3. Results and Discussion
3.1. Physico-Chemical Characterization
3.1.1. Water Uptake Behavior
3.1.2. Swelling and Degradation Behavior
3.1.3. FTIR
3.1.4. SEM Analysis
3.1.5. Nano Indentation
3.2. Effect of Incorporation of Bioactive Glass Nanoparticles into the Alginate/SPI Films
3.2.1. Water Uptake
3.2.2. Swelling and Degradation Behavior
3.3. Cell-Material Interactions Using Fibroblasts and HUVECs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Nicodemus, G.D.; Bryant, S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. Part B Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, T.; Sarker, B.; Boccaccini, A.R.; Detsch, R. Evaluation of an alginate-gelatine crosslinked hydrogel for bioplotting. Biofabrication 2015, 7, 025001. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Boontheekul, T.; Kong, H.J.; Mooney, D.J. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 2005, 26, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Reakasame, S.; Boccaccini, A.R. Oxidized alginate based hydrogels for tissue engineering applications: A review. Biomacromolecules 2017. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.S.; Santos, M.I.; Coutinho, O.P.; Mano, J.F.; Reis, R.L. Physical properties and biocompatibility of chitosan/soy blended membranes. J. Mater. Sci. Mater. Med. 2005, 16, 575–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tansaz, S.; Durmann, A.-K.; Detsch, R.; Boccaccini, A.R. Hydrogel films and microcapsules based on soy protein isolate combined with alginate. J. Appl. Polym. Sci. 2017, 134, 1–9. [Google Scholar] [CrossRef]
- Francis, L.; Meng, D.; Locke, I.C.; Knowles, J.C.; Mordan, N.; Salih, V.; Boccaccini, A.R.; Roy, I. Novel poly(3-hydroxybutyrate) composite films containing bioactive glass nanoparticles for wound healing applications. Polym. Int. 2016, 65, 661–674. [Google Scholar] [CrossRef] [Green Version]
- Naseri, S.; Lepry, W.C.; Nazhat, S.N. Bioactive glasses in wound healing: Hope or hype? J. Mater. Chem. B 2017. [Google Scholar] [CrossRef]
- Miguez-Pacheco, V.; Hench, L.L.; Boccaccini, A.R. Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 2015, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ostomel, T.A.; Shi, Q.; Tsung, C.K.; Liang, H.; Stucky, G.D. Spherical bioactive glass with enhanced rates of hydroxyapatite deposition and hemostatic activity. Small 2006, 2, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Tansaz, S.; Schulte, M.; Kneser, U.; Mohn, D.; Stark, W.; Roether, J.A.; Cicha, I.; Boccaccini, A.R. Soy protein isolate/bioactive glass composite membranes: Processing and properties. Eur. Polym. J. 2018. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Sameni, M.; Hashemi, A.; Zamani, F.; Rostami, A.; Mozafari, M. Silver- and fluoride-containing mesoporous bioactive glasses versus commonly used antibiotics: Activity against multidrug-resistant bacterial strains isolated from patients with burns. Burns 2016, 42, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Gorustovich, A.A.; Roether, J.A.; Boccaccini, A.R. Effect of bioactive glasses on angiogenesis: A review of in vitro and in vivo evidences. Tissue Eng. Part B Rev. 2010, 16, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Jebahi, S.; Oudadesse, H.; Jardak, N.; Khayat, I.; Keskes, H.; Khabir, A.; Rebai, T.; El Feki, H.; El Feki, A. Biological therapy of strontium-substituted bioglass for soft tissue wound-healing: Responses to oxidative stress in ovariectomised rats. Ann. Pharm. Fr. 2013, 71, 234–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooperman, L.; Michaeli, D. The immunogenicity of injectable collagen. I. A 1-year prospective study. J. Am. Acad. Dermatol. 1984, 10, 638–646. [Google Scholar] [CrossRef]
- DeLustro, F.; Condell, R.A.; Nguyen, M.A.; McPherson, J.M. A comparative study of the biologic and immunologic response to medical devices derived from dermal collagen. J. Biomed. Mater. Res. 1986, 20, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.Y.; Glattauer, V.; Ramshaw, J.A.M.; Werkmeister, J.A. Evaluation of the immunogenicity and cell compatibility of avian collagen for biomedical applications. J. Biomed. Mater. Res. A 2010, 93, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Cooperman, L.; Michaeli, D.; Alto, P.; Francisco, S.; Corporation, C.; Corpora, C. The immunogenicity of injectable collagen. II. A retrospective review of seventy-two tested and treated patients. J. Am. Acad. Dermatol. 1984, 10, 647–651. [Google Scholar] [CrossRef]
- Brunner, T.J.; Grass, R.N.; Stark, W.J. Glass and bioglass nanopowders by flame synthesis. Chem. Commun. 2006, 1384–1386. [Google Scholar] [CrossRef] [PubMed]
- Strobel, L.A.; Hild, N.; Mohn, D.; Stark, W.J.; Hoppe, A.; Gbureck, U.; Horch, R.E.; Kneser, U.; Boccaccini, A.R. Novel strontium-doped bioactive glass nanoparticles enhance proliferation and osteogenic differentiation of human bone marrow stromal cells. J. Nanoparticle Res. 2013, 15. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.; Bulut, B.; Roether, J.A.; Kaschta, J.; Schubert, D.W.; Boccaccini, A.R. Sonochemical processing and characterization of composite materials based on soy protein and alginate containing micron-sized bioactive glass particles. J. Mol. Struct. 2014, 1073, 87–96. [Google Scholar] [CrossRef]
- Wiebe, J.P.; Dinsdale, C.J. Inhibition of cell proliferation by glycerol. Life Sci. 1991, 48, 1511–1517. [Google Scholar] [CrossRef]
- Nguyen, T.-P.; Lee, B.-T. Fabrication of oxidized alginate-gelatin-BCP hydrogels and evaluation of the microstructure, material properties and biocompatibility for bone tissue regeneration. J. Biomater. Appl. 2012, 27, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Singh, R.; Sarker, B.; Papageorgiou, D.G.; Juhasz, J.A.; Roether, J.A.; Cicha, I.; Kaschta, J.; Schubert, D.; Chrissafis, K.; et al. Hybrid hydrogels based on keratin and alginate for tissue engineering. J. Mater. Chem. B 2014, 2, 5441. [Google Scholar] [CrossRef]
- Silva, R.; Singh, R.; Sarker, B.; Papageorgiou, D.G.; Juhasz, J.A.; Roether, J.A.; Cicha, I.; Kaschta, J.; Schubert, D.W.; Chrissafis, K.; et al. Soft-matrices based on silk fibroin and alginate for tissue engineering. Int. J. Biol. Macromol. 2016, 2, 5441–5451. [Google Scholar] [CrossRef] [PubMed]
- Leite, Á.J.; Sarker, B.; Zehnder, T.; Silva, R.; Mano, J.F.; Boccaccini, A.R. Bioplotting of a bioactive alginate dialdehyde-gelatin composite hydrogel containing bioactive glass nanoparticles. Biofabrication 2016, 8, 035005. [Google Scholar] [CrossRef] [PubMed]
- Cicha, I.; Goppelt-Struebe, M.; Muehlich, S.; Yilmaz, A.; Raaz, D.; Daniel, W.G.; Garlichs, C.D. Pharmacological inhibition of RhoA signaling prevents connective tissue growth factor induction in endothelial cells exposed to non-uniform shear stress. Atherosclerosis 2008, 196, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.A.; Shin, M.S.; Yang, J.W. Preparation and characterization of hydrophobically modified alginate. Polym. Bull. 2002, 47, 429–435. [Google Scholar] [CrossRef]
- Saarai, A.; Kasparkova, V.; Sedlacek, T.; Saha, P. On the development and characterisation of crosslinked sodium alginate/gelatine hydrogels. J. Mech. Behav. Biomed. Mater. 2013, 18, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.F.; Cestari, A.R.; Airoldi, C.; Loh, W. Polysaccharide-based hydrogels: Preparation, characterization, and drug interaction behaviour. Biomacromolecules 2008, 9, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Haris, P.I.; Severcan, F. FTIR spectroscopic characterization of protein structure in aqueous and non-aqueous media. J. Mol. Catal.–B Enzym. 1999, 7, 207–221. [Google Scholar] [CrossRef]
- Chittur, K.K. FTIR/ATR for protein adsorption to biomaterial surfaces. Biomaterials 1998, 19, 357–369. [Google Scholar] [CrossRef]
- Dev, S.B.; Keller, J.T.; Rha, C.K. Secondary structure of 11 S globulin in aqueous solution investigated by FT-IR derivative spectroscopy. Biochim. Biophys. Acta–Protein Struct. Mol. Enzymol. 1988, 957, 272–280. [Google Scholar] [CrossRef]
- Kokabi, M.; Sirousazar, M.; Hassan, Z.M. PVA–clay nanocomposite hydrogels for wound dressing. Eur. Polym. J. 2007, 43, 773–781. [Google Scholar] [CrossRef]
- Day, R.M. Bioactive Glass Stimulates the Secretion of Angiogenic Growth Factors and Angiogenesis in Vitro. Tissue Eng. 2005, 11, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Keshaw, H.; Forbes, A.; Day, R.M. Release of angiogenic growth factors from cells encapsulated in alginate beads with bioactive glass. Biomaterials 2005, 26, 4171–4179. [Google Scholar] [CrossRef] [PubMed]
- Arkudas, A.; Balzer, A.; Buehrer, G.; Arnold, I.; Hoppe, A.; Detsch, R.; Newby, P.; Fey, T.; Greil, P.; Horch, R.E.; et al. Evaluation of Angiogenesis of Bioactive Glass in the Arteriovenous Loop Model. Tissue Eng. Part C Methods 2013, 19, 479–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, R.M.; Boccaccini, A.R.; Shurey, S.; Roether, J.A.; Forbes, A.; Hench, L.L.; Gabe, S.M. Assessment of polyglycolic acid mesh and bioactive glass for soft-tissue engineering scaffolds. Biomaterials 2004, 25, 5857–5866. [Google Scholar] [CrossRef] [PubMed]
- Day, R.M.; Maquet, V.; Boccaccini, A.R.; Jérôme, R.; Forbes, A. In vitro and in vivo analysis of macroporous biodegradable poly(D,L-lactide-co-glycolide) scaffolds containing bioactive glass. J. Biomed. Mater. Res.–Part A 2005, 75, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, A.S.; Hinton, B.; Van Vliet, K.J. Why the dish makes a difference: Quantitative comparison of polystyrene culture surfaces. Acta Biomater. 2013, 9, 7354–7361. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, C.P.; Dolatshahi-Pirouz, A.; Foss, M.; Chevallier, J.; Fink, T.; Zachar, V.; Besenbacher, F.; Yoshida, K. Nanoscale topography reduces fibroblast growth, focal adhesion size and migration-related gene expression on platinum surfaces. Colloids Surf. B Biointerfaces 2011, 85, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Sarker, B.; Silva, R.; Detsch, R.; Dietel, B.; Alexiou, C.; Boccaccini, A.R.; Cicha, I. Evaluation of hydrogel matrices for vessel bioplotting: Vascular cell growth and viability. J. Biomed. Mater. Res.–Part A 2016, 104, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Singh, R.; Sarker, B.; Papageorgiou, D.G.; Juhasz-Bortuzzo, J.A.; Roether, J.A.; Cicha, I.; Kaschta, J.; Schubert, D.W.; Chrissafis, K.; et al. Hydrogel matrices based on elastin and alginate for tissue engineering applications. Int. J. Biol. Macromol. 2018, 114, 614–625. [Google Scholar] [CrossRef] [PubMed]













| Formulation | Compound 1 | Compound 2 | Compound 3 | Final Composition |
|---|---|---|---|---|
| Alg | Alg 2.5% | - | - | 100 (2.5%) |
| Alg/SPI (70/30) | Alg 5% | SPI 2% | - | 50/50 (2.5%/1%) |
| Alg/SPI (50/50) | Alg 2% | SPI 2% | - | 50/50 (1%/1%) |
| Alg/SPI (50/50) + nBG | Alg 2% | SPI 2% | nBG | 50/50 (1%/1%) + 0.5% (w/v) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tansaz, S.; Singh, R.; Cicha, I.; Boccaccini, A.R. Soy Protein-Based Composite Hydrogels: Physico-Chemical Characterization and In Vitro Cytocompatibility. Polymers 2018, 10, 1159. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10101159
Tansaz S, Singh R, Cicha I, Boccaccini AR. Soy Protein-Based Composite Hydrogels: Physico-Chemical Characterization and In Vitro Cytocompatibility. Polymers. 2018; 10(10):1159. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10101159
Chicago/Turabian StyleTansaz, Samira, Raminder Singh, Iwona Cicha, and Aldo R. Boccaccini. 2018. "Soy Protein-Based Composite Hydrogels: Physico-Chemical Characterization and In Vitro Cytocompatibility" Polymers 10, no. 10: 1159. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10101159