Segregation versus Interdigitation in Highly Dynamic Polymer/Surfactant Layers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Tensiometry
2.2.2. Foam Stability Measurements
2.2.3. Small-Angle Neutron Scattering (SANS)
SANS Data Modelling
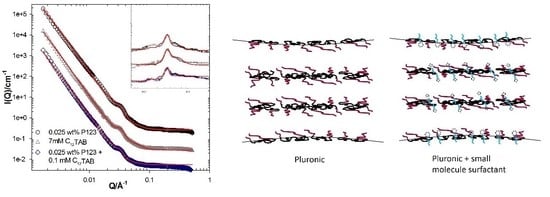
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Weaire, D.; Hutzler, S. The Physics of Foams; Oxford University Press: Oxford, UK, 1999; ISBN 0198505515. [Google Scholar]
- Langevin, D. Complexation of oppositely charged polyelectrolytes and surfactants in aqueous solutions. A review. Adv. Colloid Interface Sci. 2009, 147–148, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.A.; Yanez Arteta, M.; Angus-Smyth, A.; Nylander, T.; Varga, I. Multilayers at interfaces of an oppositely charged polyelectrolyte/ surfactant system resulting from the transport of bulk aggregates under gravity. J. Phys. Chem. B 2012, 116, 7981–7990. [Google Scholar] [CrossRef] [PubMed]
- Angus-Smyth, A.; Campbell, R.A.; Bain, C.D. Dynamic adsorption of weakly interacting polymer/surfactant mixtures at the air/water interface. Langmuir 2012, 28, 12479–12492. [Google Scholar] [CrossRef] [PubMed]
- Varga, I.; Campbell, R.A. General physical description of the behavior of oppositely charged polyelectrolyte/surfactant mixtures at the air/water interface. Langmuir 2017, 33, 5915–5924. [Google Scholar] [CrossRef] [PubMed]
- Llamas, S.; Fernández-Penã, L.; Akanno, A.; Guzmán, E.; Ortega, V.; Ortega, F.; Csaky, A.G.; Campbell, R.A.; Rubio, R.G. Towards understanding the behavior of polyelectrolyte-surfactant mixtures at the water/vapor interface closer to technologically-relevant conditions. Phys. Chem. Chem. Phys. 2018, 20, 1395–1407. [Google Scholar] [CrossRef]
- Zhang, X.L.; Taylor, D.J.F.; Thomas, R.K.; Penfold, J. Adsorption of Polyelectrolyte/Surfactant Mixtures at the Air-Water Interface: Modified Poly (ethyleneimine) and Sodium Dodecyl Sulfate. Langmuir 2011, 27, 2601–2612. [Google Scholar] [CrossRef] [PubMed]
- Halacheva, S.S.; Penfold, J.; Thomas, R.K.; Webster, J.R.P. Solution pH and oligoamine molecular weight dependence of the transition from monolayer to multilayer adsorption at the air-water interface from sodium dodecyl sulfate/oligoamine mixtures. Langmuir 2013, 29, 5832–5840. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.D.; Claesson, P.M.; Langevin, D.; Meszaros, R.; Nylander, T.; Stubenrauch, C.; Titmuss, S.; von Klitzing, R. Complexes of surfactants with oppositely charged polymers at surfaces and in bulk. Adv. Colloid Interface Sci. 2010, 155, 32–49. [Google Scholar] [CrossRef]
- Campbell, R.A.; Yanez Arteta, M.; Angus-Smyth, A.; Nylander, T.; Noskov, B.A.; Varga, I. Direct impact of nonequilibrium aggregates on the structure and morphology of pdadmac/SDS layers at the air/water interface. Langmuir 2014, 30, 8664–8674. [Google Scholar] [CrossRef]
- Campbell, R.A.; Yanez Arteta, M.; Angus-Smyth, A.; Nylander, T.; Varga, I. Effects of bulk colloidal stability on adsorption layers of poly(diallyldimethylammonium chloride)/sodium dodecyl sulfate at the air-water interface studied by neutron reflectometry. J. Phys. Chem. B 2011, 115, 15202–15213. [Google Scholar] [CrossRef]
- Petkova, R.; Tcholakova, S.; Denkov, N.D. Foaming and foam stability for mixed polymer-surfactant solutions: Effects of surfactant type and polymer charge. Langmuir 2012, 28, 4996–5009. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.B.; Li, Z.X.; Thomas, R.K.; Penfold, J. Structure of triblock copolymers of ethylene oxide and propylene oxide at the air/water interface determined by neutron reflection. J. Phys. Chem. B 2002, 106, 10641–10648. [Google Scholar] [CrossRef]
- Vieira, J.B.; Thomas, R.K.; Li, Z.X.; Penfold, J. Unusual micelle and surface adsorption behavior in mixtures of surfactants with an ethylene oxide-propylene oxide triblock copolymer. Langmuir 2005, 21, 4441–4451. [Google Scholar] [CrossRef]
- Sedev, R.; Steitz, R.; Findenegg, G.H. The structure of PEO–PPO–PEO triblock copolymers at the water/air interface. Phys. B 2002, 315, 267–272. [Google Scholar] [CrossRef]
- Braun, L.; Uhlig, M.; von Klitzing, R.; Campbell, R.A. Polymers and surfactants at fluid interfaces studied with specular neutron reflectometry. Adv. Colloid Interface Sci. 2017, 247, 130–148. [Google Scholar] [CrossRef] [PubMed]
- Halacheva, S.S.; Penfold, J.; Thomas, R.K. Adsorption of the Linear Poly(ethyleneimine) Precursor Poly(2-ethyl-2-oxazoline) and Sodium Dodecyl Sulfate Mixtures at the Air-Water Interface: The Impact of Modification of the Poly(ethyleneimine) Functionality. Langmuir 2012, 28, 17331–17338. [Google Scholar] [CrossRef] [PubMed]
- Halacheva, S.S.; Penfold, J.; Thomas, R.K.; Webster, J.R.P. Effect of architecture on the formation of surface multilayer structures at the air-solution interface from mixtures of surfactant with small poly(ethyleneimine)s. Langmuir 2012, 28, 6336–6347. [Google Scholar] [CrossRef]
- Taylor, D.J.F.; Thomas, R.K.; Penfold, J. The adsorption of oppositely charged polyelectrolyte/surfactant mixtures: Neutron reflection from dodecyl trimethylammonium bromide and sodium poly(styrene sulfonate) at the air/water interface. Langmuir 2002, 18, 4748–4757. [Google Scholar] [CrossRef]
- Taylor, D.J.F.; Thomas, R.K.; Li, P.X.; Penfold, J. Adsorption of oppositely charged polyelectrolyte/surfactant mixtures. Neutron reflection from alkyl trimethylammonium bromides and sodium poly(styrenesulfonate) at the air/water interface: The effect of surfactant chain length. Langmuir 2003, 19, 3712–3719. [Google Scholar] [CrossRef]
- Hurcom, J.; Paul, A.; Heenan, R.K.; Davies, A.; Woodman, N.; Schweins, R.; Griffiths, P.C. The interfacial structure of polymeric surfactant stabilised air-in-water foams. Soft Matter 2014, 10, 3003–3008. [Google Scholar] [CrossRef] [Green Version]
- Mansour, O.T.; Cattoz, B.; Beaube, M.; Montagnon, M.; Heenan, R.K.; Schweins, R.; Appavou, M.-S.; Griffiths, P.C. Assembly of small molecule surfactants at highly dynamic air–water interfaces. Soft Matter 2017, 13, 8807–8815. [Google Scholar] [CrossRef] [PubMed]
- Curschellas, C.; Kohlbrecher, J.; Geue, T.; Fischer, P.; Schmitt, B.; Rouvet, M.; Windhab, E.J.; Limbach, H.J. Foams stabilized by multilamellar polyglycerol ester self-assemblies. Langmuir 2013, 29, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mikhailovskaya, A.; Yazhgur, P.; Muller, F.; Cousin, F.; Langevin, D.; Wang, N.; Salonen, A. Precipitating Sodium Dodecyl Sulfate to Create Ultrastable and Stimulable Foams. Angew. Chemie-Int. Ed. 2015, 54, 9533–9536. [Google Scholar] [CrossRef] [PubMed]
- Ederth, T.; Thomas, R.K. A neutron reflectivity study of drainage and stratification of AOT foam films. Langmuir 2003, 19, 7727–7733. [Google Scholar] [CrossRef]
- Kotlarchyk, M.; Ritzau, S.M. Paracrystal model of the high-temperature lamellar phase of a ternary microemulsion system. J. Appl. Crystallogr. 1991, 24, 753–758. [Google Scholar] [CrossRef] [Green Version]
- Shibayama, M.; Hashimoto, T. Small-Angle X-ray Scattering Analyses of Lamellar Microdomains Based on a Model of One-Dimensional Paracrystal with Uniaxial Orientation. Macromolecules 1986, 19, 740–749. [Google Scholar] [CrossRef]
- Ropers, M.H.; Novales, B.; Boué, F.; Axelos, M. Polysaccharide/Surfactant complexes at the air-water interface-effect of the charge density on interfacial and foaming behaviors. Langmuir 2008, 24, 12849–12857. [Google Scholar] [CrossRef]
- Schmidt, I.; Novales, B.; Boué, F.; Axelos, M. Foaming properties of protein/pectin electrostatic complexes and foam structure at nanoscale. J. Colloid Interface Sci. 2010, 345, 316–324. [Google Scholar] [CrossRef] [Green Version]
- Etrillard, J.; Axelos, M.A.V.; Cantat, I.; Artzner, F.; Renault, A.; Weiss, T.; Delannay, R. In Situ Investigations on Organic Foam Films Using Neutron and Synchrotron Radiation. Langmuir 2005, 21, 2229–2234. [Google Scholar] [CrossRef]
- Fameau, A.L.; Saint-Jalmes, A.; Cousin, F.; Houinsou Houssou, B.; Novales, B.; Navailles, L.; Nallet, F.; Gaillard, C.; Boué, F.; Douliez, J.P. Smart foams: Switching reversibly between ultrastable and unstable foams. Angew. Chemie-Int. Ed. 2011, 50, 8264–8269. [Google Scholar] [CrossRef]
- Micheau, C.; Bauduin, P.; Diat, O.; Faure, S. Specific salt and pH effects on foam film of a pH sensitive surfactant. Langmuir 2013, 29, 8472–8481. [Google Scholar] [CrossRef]
- Mata, J.; Joshi, T.; Varade, D.; Ghosh, G.; Bahadur, P. Aggregation behavior of a PEO–PPO–PEO block copolymer + ionic surfactants mixed systems in water and aqueous salt solutions. Colloids Surfaces A Physicochem. Eng. Asp. 2004, 247, 1–7. [Google Scholar] [CrossRef]
- Mansour, O.T.; Cattoz, B.; Heenan, R.K.; King, S.M.; Griffiths, P.C. Probing competitive interactions in quaternary formulations. J. Colloid Interface Sci. 2015, 454, 35–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, R.K.; Penfold, J. Multilayering of Surfactant Systems at the Air-Dilute Aqueous Solution Interface. Langmuir 2015, 31, 7440–7456. [Google Scholar] [CrossRef] [PubMed]
- Bahramian, A.; Thomas, R.K.; Penfold, J. The adsorption behavior of ionic surfactants and their mixtures with nonionic polymers and with polyelectrolytes of opposite charge at the air-water interface. J. Phys. Chem. B 2014, 118, 2769–2783. [Google Scholar] [CrossRef] [PubMed]
- Briddick, A.; Fong, R.; Sabattie, E.; Li, P.; Skoda, M.W.A.; Courchay, F.; Thompson, R.L. Blooming of Smectic Surfactant/Plasticizer Layers on Spin-Cast Poly(vinyl alcohol) Films. Langmuir 2018, 34, 1410–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| System Description | L (Å) ± 2 | M | D (Å) ± 5 |
|---|---|---|---|
| 0.025 wt % P123 (D11) | 140 | 4 | 180 |
| 0.025 wt % P123 (SANS2d) | 140 | 5 | 390 |
| 7 mM C12TAB | 40 | 5 | 180 |
| 0.025 wt % P123 + 0.1 mM C12TAB | 140 | 4 | 180 |
| 4 mM SDS | 35 | 5 | 180 |
| 0.025 wt % P123 + 0.1 mM SDS | 120 | 5 | 410 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansour, O.T.; Cattoz, B.; Beaube, M.; Heenan, R.K.; Schweins, R.; Hurcom, J.; Griffiths, P.C. Segregation versus Interdigitation in Highly Dynamic Polymer/Surfactant Layers. Polymers 2019, 11, 109. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11010109
Mansour OT, Cattoz B, Beaube M, Heenan RK, Schweins R, Hurcom J, Griffiths PC. Segregation versus Interdigitation in Highly Dynamic Polymer/Surfactant Layers. Polymers. 2019; 11(1):109. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11010109
Chicago/Turabian StyleMansour, Omar T., Beatrice Cattoz, Manon Beaube, Richard K. Heenan, Ralf Schweins, Jamie Hurcom, and Peter C. Griffiths. 2019. "Segregation versus Interdigitation in Highly Dynamic Polymer/Surfactant Layers" Polymers 11, no. 1: 109. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11010109