Improving the Performances of Perovskite Solar Cells via Modification of Electron Transport Layer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Device Fabrication
2.3. Device Characterization
3. Results
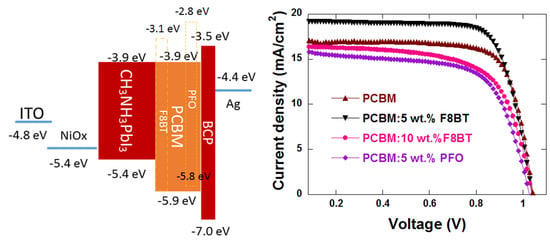
3.1. Device Performance
3.2. Film Properties
3.3. Charge Carrier Dynamic
3.4. Energy Level Matching
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stolterfoht, M.; Wolff, C.M.; Marquez, J.A.; Zhang, S.S.; Hages, C.J.; Rothhardt, D.; Albrecht, S.; Burn, P.L.; Meredith, P.; Unold, T.; et al. Visualization and suppression of interfacial recombination for high-efficiency large-area pin perovskite solar cells. Nat. Energy 2018, 3, 847–854. [Google Scholar] [CrossRef]
- Park, B.W.; Kedem, N.; Kulbak, M.; Lee, D.Y.; Yang, W.S.; Jeon, N.J.; Seo, J.; Kim, G.; Kim, K.J.; Shin, T.J.; et al. Understanding how excess lead iodide precursor improves halide perovskite solar cell performance. Nat. Commun. 2018, 9, 3301. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.Q.; Li, X.; Milic, J.V.; Kubicki, D.J.; Pellet, N.; Luo, J.S.; Lagrange, T.; Mettraux, P.; Emsley, L.; Zakeeruddin, S.M.; et al. Multifunctional molecular modulators for perovskite solar cells with over 20% efficiency and high operational stability. Nat. Commun. 2018, 9, 4482. [Google Scholar] [CrossRef]
- Tsai, C.L.; Lu, Y.C.; Chang, S.H. Enhancement of photocurrent extraction and electron injection in dual-functional CH3NH3PbBr3 perovskite-based optoelectronic devices via interfacial engineering. Nanotechnology 2018, 29, 275704. [Google Scholar] [CrossRef] [PubMed]
- Li, D.M.; Shi, J.J.; Xu, Y.Z.; Luo, Y.H.; Wu, H.J.; Meng, Q.B. Inorganic-organic halide perovskites for new photovoltaic technology. Natl. Sci. Rev. 2018, 5, 559–576. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.W.; Wang, C.L.; Song, Z.N.; Yu, Y.; Chen, C.; Zhao, X.Z.; Zhu, K.; Yan, Y.F. Four-Terminal All-Perovskite Tandem Solar Cells Achieving Power Conversion Efficiencies Exceeding 23%. ACS Energy Lett. 2018, 3, 305–306. [Google Scholar] [CrossRef]
- Cho, K.T.; Zhang, Y.; Orlandi, S.; Cavazzini, M.; Zimmermann, I.; Lesch, A.; Tabet, N.; Pozzi, G.; Grancini, G.; Nazeeruddin, M.K. Water-Repellent Low-Dimensional Fluorous Perovskite as Interfacial Coating for 20% Efficient Solar Cells. Nano Lett. 2018, 18, 5467–5474. [Google Scholar] [CrossRef]
- Saliba, M.; Correa-Baena, J.P.; Wolff, C.M.; Stolterfoht, M.; Phung, N.; Albrecht, S.; Neher, D.; Abate, A. How to Make over 20% Efficient Perovskite Solar Cells in Regular (n-i-p) and Inverted (p-i-n) Architectures. Chem. Mater. 2018, 30, 4193–4201. [Google Scholar] [CrossRef]
- Momblona, C.; Gil-Escrig, L.; Bandiello, E.; Hutter, E.M.; Sessolo, M.; Lederer, K.; Blochwitz-Nimoth, J.; Bolink, H.J. Efficient vacuum deposited p-i-n and n-i-p perovskite solar cells employing doped charge transport layers. Energy Environ. Sci. 2016, 9, 3456–3463. [Google Scholar] [CrossRef]
- Huang, J.; Wang, K.X.; Chang, J.J.; Jiang, Y.Y.; Xiao, Q.S.; Li, Y. Improving the efficiency and stability of inverted perovskite solar cells with dopamine-copolymerized PEDOT: PSS as a hole extraction layer. J. Mater. Chem. A 2017, 5, 13817–13822. [Google Scholar] [CrossRef]
- Zeng, X.F.; Zhou, T.W.; Leng, C.Q.; Zang, Z.G.; Wang, M.; Hu, W.; Tang, X.S.; Lu, S.R.; Fang, L.A.; Zhou, M. Performance improvement of perovskite solar cells by employing a CdSe quantum dot/PCBM composite as an electron transport layer. J. Mater. Chem. A 2017, 5, 17499–17505. [Google Scholar] [CrossRef]
- Fu, X.M.; Xu, L.M.; Li, J.X.; Sun, X.M.; Peng, H.S. Flexible solar cells based on carbon nanomaterials. Carbon 2018, 139, 1063–1073. [Google Scholar] [CrossRef]
- Abate, A.; Correa-Baena, J.P.; Saliba, M.; Su’ait, M.S.; Bella, F. Perovskite Solar Cells: From the Laboratory to the Assembly Line. Chem.-Eur. J. 2018, 24, 3083–3100. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Yan, K.; Tan, D.W.; Liang, X.; Zhang, H.M.; Huang, W. Solvent Engineering Improves Efficiency of Lead-Free Tin-Based Hybrid Perovskite Solar Cells beyond 9%. ACS Energy Lett. 2018, 3, 2701–2707. [Google Scholar] [CrossRef]
- Xing, G.C.; Mathews, N.; Sun, S.Y.; Lim, S.S.; Lam, Y.M.; Gratzel, M.; Mhaisalkar, S.; Sum, T.C. Long-Range Balanced Electron- and Hole-Transport Lengths in Organic-Inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef]
- Xiao, Z.G.; Bi, C.; Shao, Y.C.; Dong, Q.F.; Wang, Q.; Yuan, Y.B.; Wang, C.G.; Gao, Y.L.; Huang, J.S. Efficient, high yield perovskite photovoltaic devices grown by interdiffusion of solution-processed precursor stacking layers. Energy Environ. Sci. 2014, 7, 2619–2623. [Google Scholar] [CrossRef]
- Liu, J.; Wang, G.; Luo, K.; He, X.L.; Ye, Q.Y.; Liao, C.; Mei, J. Understanding the Role of the Electron-Transport Layer in Highly Efficient Planar Perovskite Solar Cells. Chemphyschem 2017, 18, 617–625. [Google Scholar] [CrossRef]
- Seo, J.; Park, S.; Kim, Y.C.; Jeon, N.J.; Noh, J.H.; Yoon, S.C.; Sang, S.I. Benefits of very thin PCBM and LiF layers for solution-processed p-i-n perovskite solar cells. Energy Environ. Sci. 2014, 7, 2642–2646. [Google Scholar] [CrossRef]
- Docampo, P.; Ball, J.M.; Darwich, M.; Eperon, G.E.; Snaith, H.J. Efficient organometal trihalide perovskite planar-heterojunction solar cells on flexible polymer substrates. Nat. Commun. 2013, 4, 2761. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.D.; Yu, H.; Yan, L.; Dong, Q.Q.; Wan, Q.; Zhou, Y.; Song, B.; Li, Y.F. Triple Cathode Buffer Layers Composed of PCBM, C-60, and LiF for High-Performance Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 6230–6237. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.F.; Hu, Z.C.; Liu, J.; Lin, J.H.; Sun, C.; Chen, Z.M.; Duan, C.H.; Wang, J.; Liao, C.; Lau, W.M.; et al. Highly efficient fullerene/perovskite planar heterojunction solar cells via cathode modification with an amino-functionalized polymer interlayer. J. Mater. Chem. A 2014, 2, 19598–19603. [Google Scholar] [CrossRef]
- Sun, K.; Chang, J.J.; Isikgor, F.H.; Li, P.C.; Ouyang, J.Y. Efficiency enhancement of planar perovskite solar cells by adding zwitterion/LiF double interlayers for electron collection. Nanoscale 2015, 7, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Zhang, Z.G.; Hou, Y.; Quiroz, C.O.R.; Przybilla, T.; Bronnbauer, C.; Guo, F.; Forberich, K.; Azimi, H.; Ameri, T.; et al. Interface Engineering of Perovskite Hybrid Solar Cells with Solution-Processed Perylene-Diimide Heterojunctions toward High Performance. Chem. Mater. 2015, 27, 227–234. [Google Scholar] [CrossRef]
- Zhang, H.; Azimi, H.; Hou, Y.; Ameri, T.; Przybilla, T.; Spiecker, E.; Kraft, M.; Scherf, U.; Brabec, C.J. Improved High-Efficiency Perovskite Planar Heterojunction Solar Cells via Incorporation of a Polyelectrolyte Interlayer. Chem. Mater. 2014, 26, 5190–5193. [Google Scholar] [CrossRef]
- Kakavelakis, G.; Maksudov, T.; Konios, D.; Paradisanos, I.; Kioseoglou, G.; Stratakis, E.; Kymakis, E. Efficient and Highly Air Stable Planar Inverted Perovskite Solar Cells with Reduced Graphene Oxide Doped PCBM Electron Transporting Layer. Adv. Energy Mater. 2017, 7, 1602120. [Google Scholar] [CrossRef]
- Huang, W.J.; Huang, P.H.; Yang, S.H. PCBM doped with fluorene-based polyelectrolytes as electron transporting layers for improving the performance of planar heterojunction perovskite solar cells. Chem. Commun. 2016, 52, 13572–13575. [Google Scholar] [CrossRef]
- Kuang, C.Y.; Tang, G.; Jiu, T.G.; Yang, H.; Liu, H.B.; Li, B.R.; Luo, W.N.; Li, X.D.; Zhang, W.J.; Lu, F.S.; et al. Highly Efficient Electron Transport Obtained by Doping PCBM with Graphdiyne in Planar-Heterojunction Perovskite Solar Cells. Nano Lett. 2015, 15, 2756–2762. [Google Scholar] [CrossRef]
- Xia, F.; Wu, Q.L.; Zhou, P.C.; Li, Y.; Chen, X.; Liu, Q.; Zhu, J.; Dai, S.Y.; Lu, Y.L.; Yang, S.F. Efficiency Enhancement of Inverted Structure Perovskite Solar Cells via Oleamide Doping of PCBM Electron Transport Layer. ACS Appl. Mater. Interfaces 2015, 7, 13659–13665. [Google Scholar] [CrossRef]
- Bai, Y.; Yu, H.; Zhu, Z.L.; Jiang, K.; Zhang, T.; Zhao, N.; Yang, S.H.; Yan, H. High performance inverted structure perovskite solar cells based on a PCBM:polystyrene blend electron transport layer. J. Mater. Chem. A 2015, 3, 9098–9102. [Google Scholar] [CrossRef]
- Niu, Q.L.; Zhou, Y.; Wang, L.; Peng, J.; Wang, J.; Pei, J.; Cao, Y. Enhancing the performance of polymer light-emitting diodes by integrating self-assembled organic nanowires. Adv. Mater. 2008, 20, 964–969. [Google Scholar] [CrossRef]
- Niu, Q.L.; Xu, Y.H.; Jiang, J.X.; Peng, J.B.; Cao, Y. Efficient polymer white-light-emitting diodes with a single-emission layer of fluorescent polymer blend. J. Lumin. 2007, 126, 531–535. [Google Scholar] [CrossRef]
- Suzuki, A.; Miyaura, N. The Cross-Coupling Reaction Between Organoboron Compounds And Organic Halides. Abstr. Pap. Am. Chem. Soc. 1995, 209. [Google Scholar] [CrossRef]
- Yin, X.T.; Chen, P.; Que, M.D.; Xing, Y.L.; Que, W.X.; Niu, C.M.; Shao, J.Y. Highly Efficient Flexible Perovskite Solar Cells Using Solution-Derived NiOx Hole Contacts. ACS Nano 2016, 10, 3630–3636. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.L.; Shao, Y.X.; Xu, W.; Wang, L.; Han, S.H.; Liu, N.H.; Peng, J.B.; Cao, Y.; Wang, J. Full color and monochrome passive-matrix polymer light-emitting diodes flat panel displays made with solution processes. Org. Electron. 2008, 9, 95–100. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Niu, Q.L.; Tang, X.; Wang, H.P.; Huang, W.T.; Xia, R.D.; Zeng, W.J.; Yao, J.Z.; Yan, B.Y. Understanding the Effect of Delay Time of Solvent Washing on the Performances of Perovskite Solar Cells. ACS Omega 2017, 2, 7666–7671. [Google Scholar] [CrossRef]
- Zhu, Z.L.; Bai, Y.; Zhang, T.; Liu, Z.K.; Long, X.; Wei, Z.H.; Wang, Z.L.; Zhang, L.X.; Wang, J.N.; Yan, F.; et al. High-Performance Hole-Extraction Layer of Sol-Gel-Processed NiO Nanocrystals for Inverted Planar Perovskite Solar Cells. Angew. Chem. Int. Ed. 2014, 53, 12571–12575. [Google Scholar]
- Al-Asbahi, B.A. Influence of SiO2/TiO2 Nanocomposite on the Optoelectronic Properties of PFO/MEH-PPV-Based OLED Devices. Polymers-Basel 2018, 10, 800. [Google Scholar] [CrossRef]
- Li, Y.P.; Lagowski, J.B. A multi-step simulation of electron mobility in fluorene-benzothiadiazole conjugated polymer—Case study. Comput. Theor. Chem. 2011, 977, 157–162. [Google Scholar] [CrossRef]
- Niu, Q.L.; Zhang, Q.; Xu, W.D.; Jiang, Y.; Xia, R.D.; Bradley, D.D.C.; Li, D.; Wen, X.S. Solution-processed anthracene-based molecular glasses as stable blue-light-emission laser gain media. Org. Electron. 2015, 18, 95–100. [Google Scholar] [CrossRef]
- Xia, R.D.; Leem, D.S.; Kirchartz, T.; Spencer, S.; Murphy, C.; He, Z.C.; Wu, H.B.; Su, S.J.; Cao, Y.; Kim, J.S.; et al. Investigation of a Conjugated Polyelectrolyte Interlayer for Inverted Polymer:Fullerene Solar Cells. Adv. Energy Mater. 2013, 3, 718–723. [Google Scholar] [CrossRef]
- Pham, N.D.; Tiong, V.T.; Chen, P.; Wang, L.Z.; Wilson, G.J.; Bell, J.; Wang, H.X. 35 Enhanced perovskite electronic properties via a modified lead(II) chloride Lewis acid-base adduct and their effect in high-efficiency perovskite solar cells. J. Mater. Chem. A 2017, 5, 5195–5203. [Google Scholar] [CrossRef]
- Wang, C.X.; Hao, H.; Chen, S.F.; Cao, K.; Yu, H.T.; Zhang, Q.; Wan, G.J.; Shang, W.J.; Huang, W. Inverse-architecture perovskite solar cells with 5,6,11,12-tetraphenylnaphthacene as a hole conductor. RSC Adv. 2017, 7, 29944–29952. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.Q.; Nie, H.; Zhou, X.B.; Hu, S.B.; Luo, D.X.; Gao, D.Y.; Zou, J.H.; Xu, M.; Wang, L.; Zhao, Z.J.; et al. Manipulation of Charge and Exciton Distribution Based on Blue Aggregation-Induced Emission Fluorophors: A Novel Concept to Achieve High-Performance Hybrid White Organic Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 776–783. [Google Scholar] [CrossRef]
- Sousa, L.; Volpi, R.; da Silva, D.A.; Linares, M. Mobility field and mobility temperature dependence in PC61BM: A kinetic Monte-Carlo study. Chem. Phys. Lett. 2017, 689, 74–81. [Google Scholar] [CrossRef]





| ETLs Used | VOC (V) | JSC (mA·cm−2) | FF a (%) | PCE b (%) |
|---|---|---|---|---|
| PCBM c | 1.03 ± 0.01 | 17.21 ± 0.15 | 71.1 ± 0.07 | 12.6 ± 0.24 |
| PCBM: 5 wt % F8BT | 1.04 ± 0.01 | 19.28 ± 0.22 | 74.7 ± 0.21 | 15 ± 0.26 |
| PCBM: 10 wt % F8BT | 1.02 ± 0.01 | 17.13 ± 0.28 | 71.5 ± 0.63 | 12.48 ± 0.37 |
| PCBM: 5 wt % PFO | 1.03 ± 0.02 | 16.3 ± 0.24 | 64 ± 0.98 | 10.8 ± 0.27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, M.; Niu, Q.; Tang, X.; Zhang, H.; Xu, H.; Huang, W.; Yao, J.; Yan, B.; Xia, R. Improving the Performances of Perovskite Solar Cells via Modification of Electron Transport Layer. Polymers 2019, 11, 147. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11010147
Jiang M, Niu Q, Tang X, Zhang H, Xu H, Huang W, Yao J, Yan B, Xia R. Improving the Performances of Perovskite Solar Cells via Modification of Electron Transport Layer. Polymers. 2019; 11(1):147. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11010147
Chicago/Turabian StyleJiang, Mao, Qiaoli Niu, Xiao Tang, Heyi Zhang, Haowen Xu, Wentao Huang, Jizhong Yao, Buyi Yan, and Ruidong Xia. 2019. "Improving the Performances of Perovskite Solar Cells via Modification of Electron Transport Layer" Polymers 11, no. 1: 147. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11010147